Abstract
The ZnO–SiO2 xerogel photocatalyst was prepared via the sol–gel technique and applied for photodegradation of methylene blue (MB) dye. The optimum conditions for preparation of ZnO–SiO2 gel is 30:70 ZnO:SiO2 molar ratio and TEOS:C2H5OH:H2O:HNO3 is 1:16:12:0.04 molar ratios at 30°C for 30 min, at these conditions the photoactivity of ZnO–SiO2 xerogel was 99% at a surface area of 500 m2/g after 60 min. The optimum loading of ZnO–SiO2 photocatalyst was 0.050 wt% that gives 99% MB dye removal efficiency after 40 min. The overall kinetics of photodegradation of MB dye using ZnO–SiO2 photocatalyst was found to be of the first order.
Similar content being viewed by others
Introduction
With increasing demands on the coloured textile industry, a large amount of toxic wastewater is produced and released into the aqueous ecosystem. Moreover, modern dye wastewater consists of high concentrations, stable colour and a complicated composition. Hence, many traditional treatment methods are limited because of low degradation efficiencies, consumption of chemicals and the generation of secondary pollution (Height et al. 2006; Gao et al. 2011). Recently, advanced oxidation processes have been used to degrade natural and synthetic dyes efficiently. In particular, semiconductor-mediated photocatalytic oxidation can be conveniently applied towards the degradation of dye pollutants using only light, catalyst, and air (Minero et al. 2005; Meng and Juan 2008; Qourzal et al. 2009). The photocatalytic process creates an electronic charge carrier in the conduction band (e−) and an electron vacancy in the valance band (h+). Because the valence band edge of ZnO occurs at approximately 3.37 eV, the hole is a very powerful oxidising agent and is capable of oxidising a variety of organic molecules as well as generating hydroxyl radicals in water (Meng and Juan 2008; Qourzal et al. 2009; Anpo et al. 1980). Among the various semiconductors recently studied, zinc oxide (ZnO) stands out for use in decomposition of organic pollutants because of its high photosensitivity, excellent mechanical characteristics, low cost and environmentally safe nature (Singhal et al. 2008; Shen et al. 2008). The use of ZnO for photocatalytic degradation of organic pollutants has been studied extensively. Examples of such studies may include degradation of trichloroethylene (Jung et al. 1997), anthraquinone sulphonic acid (Sivakumar et al. 2000), 2-chlorophenol (Abdel Aal et al. 2008), Rhodamine dyes (Yu et al. 2004), azo-reactive dyes (Fouad et al. 2006), Congo Red (Movahedi et al. 2009), and Methylene Blue (MB) (Chu et al. 2010). However, silicon dioxide (SiO2) has been coupled with semiconductor photocatalysts to enhance the photocatalytic process. SiO2 has high thermal stability, excellent mechanical strength and helps to create new catalytic active sites due to the interaction between semiconductor photocatalysts and SiO2. Additionally, SiO2 helps to obtain a large surface area as well as a suitable porous structure (Abd Aziz and Sopyan 2009; Anderson and Bard 1995; Ruetten and Thomas 2003; Chun et al. 2001). The present study aims to determine the optimal conditions for the preparation of the ZnO–SiO2 xerogel photocatalyst via the sol–gel technique. The photocatalyst was used in the photocatalytic degradation of methylene blue.
Experimental
Materials and procedure
All chemicals used namely are: zinc nitrate hexahydrated [Zn (NO3)2 6H2O] from BDH Laboratories Supplies and tetraethylorthosilicate [TEOS, Si(OC2H5)4, 98%] from ACROS organics Laboratories Supplies. A total of 20 ml TEOS was mixed with ethyl alcohol (C2H5OH), ultra pure water (H2O) and nitric acid (HNO3) as catalyst under magnetic stirring for 60 min. Then calculated amount of Zn (NO3)2 6H2O was added simultaneously and slowly into the previous mixture with continuous stirring for 30 min. The prepared sol was left to stand the formation of gel. The gel sample was calcined at 550°C for 5 h in air to obtain the ZnO–SiO2 xerogel.
Photocatalytic evaluation
Methylene blue was selected as a model for the photocatalytic degradation experiments because it is a common contaminant in industrial textile wastewater.
All of the experiments were carried out with a horizontal cylinder annular batch reactor. A black light fluorescent bulb (F18 W-BLB) was positioned at the axis of the reactor to supply UV illumination. The wavelength of the used UV lamp was 365 nm. The experiments were performed by suspending 0.1 g of ZnO–SiO2 xerogel into the reactor with 300 ml aqueous MB solution (50 ppm). The reaction was carried out at room temperature and pH 7, and a sample of the reaction mixture was taken after 60 min for analysis.
Methylene blue content in the solution was determined by monitoring the absorbance of MB solutions using a UV–vis spectrophotometer, which was recorded on a Shimadzu UV-2450 with a 1-cm (path length) quartz cuvette. The photodegradation efficiency of MB was estimated by applying the following equation:
where C0 is the original content of MB, and C is the residual MB in solution.
Characterisation of xerogel samples
The structure of the catalyst was examined by X-ray diffraction (XRD) on a Rigaku X-ray diffractometer system equipped with as RINT 2000 wide angle Joniometer using Cu Kα radiation and a power of 40 kV × 30 mA. The intensity data were collected at 25°C over a 2θ range of 10°–80°. N2-adsorption measurement was carried out at 77 K using Nova 2000 series, Chromatech. Prior to analysis, the samples were outgased at 250°C for 4 h. The amount of ZnO and SiO2 in the crystalline samples were obtained by Energy Dispersive X-ray technique (Oxford) that combined with Scanning Electron Microscope (JEOL-JSM-5410). Particle sizes of the produced samples were recorded by Horiba Dynamic light Scattering Particle Size Analyzer LB-500.
Results and discussion
Synthesis of ZnO–SiO2 xerogel
Controlling the homogeneity of ZnO in the SiO2 matrix is very important for improving the surface area and photoactivity of the catalyst. This depends on the microstructure of ZnO–SiO2 binary xerogel, which is changed by the ZnO:SiO2 molar ratio, the TEOS:C2H5OH:H2O:HNO3 molar ratio, the reaction time, the calcination temperature, and the calcination time.
Effect of the ZnO:SiO2 molar ratio
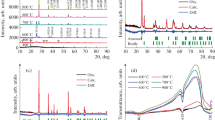
A series of experiments was carried out by changing ZnO:SiO2 molar ratio from 0.05 to 0.66. The X-ray diffraction analysis for all samples with different ZnO:SiO2 molar ratios have been performed as shown in Fig. 1. They exhibit several crystalline peaks mainly composed of the ZnO crystallites phase (PDF standard card 36-1451), and SiO2 was formed as an amorphous phase. The peak intensities of ZnO/SiO2 nanoparticles were high and sharp at high ZnO content, and they were smaller with a decrease in the ZnO:SiO2 molar ratio. This is possibly the result of the increase of SiO2 content in an amorphous state around the ZnO nanoparticles (Li et al. 2009). At low ZnO content, the peaks are broad, which suggest that the particles have sizes in the nanometer range.
The chemical composition of the prepared samples was measured by EDX technique and the data are summarised in Table 1. The results indicated that the Zn/Si atomic % at three areas is almost similar and hence the samples are homogeneous. The particle size of the optimised sample was 30 nm which measured by Horiba Dynamic light Scattering Particle Size Analyzer LB-500. Figure 2 shows the adsorption–desorption isotherm of the optimised sample. The results reveal that the type of isotherm of the optimised sample is type II.
To study the effect of the ZnO:SiO2 molar ratio on the surface area and the photodegradation efficiency of methylene blue, a series of experiments was carried out by changing ZnO:SiO2 molar ratio from 0.05 to 0.66. We found that as the ZnO:SiO2 molar ratio changed from 0.05 to 0.42, the photodegradation efficiency increased from 74.16 to 81% while surface area decreased from 400 to 320 m2/g due to the decrease in SiO2 concentration. As the ZnO:SiO2 molar ratio increased to 0.66, the surface area still decreased until it reached 290 m2/g where the photodegradation efficiency decreased to 78%, as shown in Fig. 3. These results indicated that as the amount of ZnO increased the photodegradation efficiency increased, even though the surface area is decreased. However, the decreasing efficiency at high amounts of ZnO could be attributed to the strong decrease in surface area. It should be mentioned here that the effective material for photocatalytic degradation is ZnO not SiO2 (Ismail et al. 2004). The optimal condition of the ZnO:SiO2 molar ratio was 0.42.
Effect of C2H5OH:TEOS molar ratio
The influence of the C2H5OH:TEOS molar ratio on the photodegradation efficiency of MB and the surface area was investigated by varying its ratio from 4 to 20, shown in Fig. 4. This illustrates that, as the C2H5OH:TEOS molar ratio increases from 4 to 16; the photodegradation efficiency of MB was increased from 81 to 87%. The surface area also increased from 320 to 380 m2/g, and the photodegradation efficiency decreased to 82% when the C2H5OH:TEOS molar ratio further increased to 20, and the surface area decreased to 373 m2/g. The increase in photocatalytic activity may be attributed to the increase in surface area, and the decrease at high C2H5OH content may be attributed to a decrease in the solution viscosity (Ismail et al. 2004). Based on these results, the optimum condition of the C2H5OH:TEOS molar ratio was 16, which gave an 87% photodegradation efficiency of MB at a 380 m2/g surface area.
Effect of the H2O:TEOS molar ratio
The influence of the H2O:TEOS molar ratio on the photodegradation efficiency of MB and the surface area was investigated by varying its ratio from 4 to 20. The results presented in Fig. 5 reveal that, when the H2O:TEOS molar ratio increases from 4 to 12, the specific surface area increases from 380 to 440 m2/g. At higher H2O:TEOS molar ratios (molar ratio changed from 12 to 20), however, the specific surface area is decreased to 315 m2/g. The proposed cause of this behaviour is that acid-catalysed low-water solutions produced linear polymers, and high-water solutions produced cross-linked polymers or branched-clusters, resulting in samples with small surface areas (Klein 1985; Walther et al. 1991; Yu and Wang 2000). In addition, the results presented in Fig. 4 revealed that, when the H2O:TEOS molar ratio increases from 4 to 12, the photodegradation efficiency of MB increases from 87 to 92%. However, the photodegradation efficiency decreased from 92 to 80% with an increasing H2O:TEOS molar ratio of 12–20. This is related to the decrease of the surface area of the photocatalyst at the H2O:TEOS ratio above 12 and may be attributed to the transparency of the sample to light. For example, at low-water content, the produced samples were more translucent. At high H2O:TEOS molar ratios, the siloxane bond hydrolysis shifts to the reverse reaction. The hydrolysis reaction may proceed in the reverse direction in which an alcohol molecule displaces a hydroxyl group to produce an alkoxide ligand and water as a by-product (Brinker and Scherer 1990). The optimal H2O:TEOS molar ratio was 12, which gave a 92% photodegradation efficiency of MB and a 440 m2/g surface area.
Effect of reaction time
The effect of the reaction time on the photodegradation efficiency of MB and the surface area was conducted by changing reaction time from 30 to 120 min. The results in Fig. 6 reveal that the photodegradation efficiency of MB was increased from 92 to 95% by increasing the reaction time from 30 to 60 min and the surface area from 440 to 470 m2/g. A further increase in the reaction time to 90 min slightly increased photodegradation efficiency of MB to 95.50% and to 95.55% when the reaction time was 120 min. The surface area increased very slowly from 472 to 473 m2/g, which meant that the hydrolysis and condensation reactions were completed within the first 60 min, and the growth of the particles was stopped (Ismail et al. 2003). For purposes of economy, the best reaction time was 60 min.
Effect of the calcination temperature
The calcination temperature can also have an important effect on the photodegradation efficiency of MB and surface area. This effect was studied in a series of ZnO/SiO2 nanoparticle samples calcinated for 5 h with changing temperatures from 150 to 650°C. The results presented in Fig. 7 show that, when calcination temperature increased from 150 to 550°C, the photodegradation efficiency of MB increases from 74 to 95% while the surface area decreased from 600 to 470 m2/g. Further increases in calcination temperature to 650°C led to a continued decrease in surface area to 350 m2/g, and the photodegradation efficiency of MB also decreased to 90%. The low photodegradation efficiency shown by the samples calcinated at temperatures lower than 550°C could be due to its poor degree of crystallinity, which was not compensated by the positive effect given by its high surface area. Alternatively, this decreased activity at high calcination temperatures could be explained by the strong decrease in surface area due to the aggregation of nanoparticles, leading to an increase in particle size (Sakthivel et al. 2006). It was observed that the most favourable photodegradation efficiency of MB occurs at 550°C with 470 m2/g surface area.
Effect of calcination time
Calcination time is an important parameter in the optimised ZnO–SiO2 xerogel samples. To study the effect of this parameter on the photodegradation efficiency of MB and the surface area, a series of experiments were carried out by varying the calcination time from 1 to 7 h under the aforementioned conditions. The results are presented in Fig. 8. As calcination time increased from 1 to 5 h, the photodegradation efficiency increased gradually from 80 to 95%, and the surface area increased from 310 to 470 m2/g. A further increase in the calcination time to 7 h slightly increased the photocatalytic activity to 95.50%, accompanied by a decrease in surface area to 468 m2/g. These results indicate that the water of crystallisation was removed within 5 h after the beginning of calcination at 550°C, and all of the sample was oxidised. The increasing photodegradation efficiency with increasing calcination time may be explained by the increasing crystallinity of the ZnO/SiO2 nanoparticles. The highest photocatalytic activity was observed in the sample calcinated for 7 h, but for economic purposes, the optimum calcination time was 5 h because there is no clear difference in the efficiency of the samples calcinated at 5 and 7 h.
Effect of acid catalysts
Under acidic conditions, it is likely that an ethoxide group is protonated rapidly as a first step. Electron density is withdrawn from silicon, making it more electrophilic and thus more susceptible to attack by water (Brinker and Scherer 1990). The influence of the HNO3:TEOS molar ratio on the photodegradation efficiency of MB and surface area was studied by varying its ratio from 0.01 to 0.05. The results presented in Fig. 9 show that, when the HNO3:TEOS molar ratio increased from 0.01 to 0.04, the hydrolysis and condensation rates increase, and the photodegradation efficiency of MB increased from 91 to 99% with a surface area increase of 465 to 500 m2/g. However, as the HNO3:TEOS molar ratio increased above 0.04, the photodegradation efficiency of MB decreased to reach 97%, and the surface area decreased to 485 m2/g at a 0.05 HNO3:TEOS molar ratio. This has been interpreted on the basis of the extent of hydrolysis and polymerisation (Ismail et al. 2004). The hydrolysis rate is increased by substituents that reduce steric crowding around silicon. In addition, a zinc electron-providing substituent that helps stabilise the developing positive charge should also increase the hydrolysis rate. At a higher acid volume, a decrease in the photodegradation efficiency of MB may be attributed to the amount of acid catalyst in solution, disturbing the transition state. It is known that the hydrolysis mechanism is sensitive to inductive effects (based on the consistent trends) and is apparently unaffected by the extent of alkyl substitution. Thus, an increase in the stability of the transition state will increase the reaction rate (Brinker and Scherer 1990). Therefore, the optimal HNO3:TEOS molar ratio is considered 0.04 at which 99% photodegradation efficiency of MB and a 500 m2/g surface area can be achieved Fig. 10.
ZnO–SiO2 xerogel loading
Photocatalytic degradation of MB dye in the presence of ZnO–SiO2 xerogel was carried out. The effect of loading of ZnO–SiO2 xerogel from 0016 to 0.1 wt% on MB dye removal efficiency was studied. The findings summarised in Table 2 show that the lowest loading 0.016 was achieved at MB removal efficiency of 79% after 60 min. Upon increasing xerogel loading from 0.016 to 0.033 wt%, the removal efficiency was increased to 99% after 60 min. Increasing xerogel loading from 0.033 to 0.1 wt%, led to decrease of reaction time from 60 to 40 min. 0.016 wt% is not enough to degrade all MB dye. By increasing the loading to 0.033 wt% the MB dye was decomposed in aqueous solution. This behaviour has been frequently observed in photocatalysis indicating that with sufficient loading all light is absorbed by ZnO and further addition of the catalyst cannot increase reaction. From the economic point of view, 0.050 wt% is considered the best xerogel loading.
The loss of the MB dye substrate was fitted to the corresponding expression:
where [c]0 and [c]t represent the concentration in ppm of MB dye solution at time zero and t of illumination, respectively, and k represents the apparent rate constant (min−1). Plotting log [c]t versus t in Fig. 10 gave linear relation. This indicated the photodegradation of MB dye is of the first order. The rate constant, k, increased with increasing loading of ZnO–SiO2 xerogel.
Conclusions
ZnO–SiO2 mixed oxides have been prepared via a sol–gel approach using TEOS and Zn (NO3)2·6H2O. The best conditions to obtain xerogel material for photocatalytic degradation of MB are 30:70 of the ZnO:SiO2 molar ratio and 1:16:12:0.04 of the TEOS:C2H5OH:H2O:HNO3 molar ratio at room temperature for 60 min. Furthermore, the sample was calcinated at 550°C for 5 h. Under these conditions, the photoactivity and surface area of ZnO–SiO2 were 99% and 500 m2/g, respectively, using 0.033 wt% of catalyst and after 60 min of reaction time. Increasing xerogel loading led to decrease of reaction time from 60 to 40 min. Therefore, 0.050 wt% of ZnO–SiO2 xerogel is enough for degradation about 99% after 40 min and the overall kinetic for degradation of MB dye was found to be of the first order.
References
Abd Aziz R, Sopyan I (2009) Synthesis of TiO2–SiO2 powder and thin film photocatalysts by sol-gel method. Indian J Chem 48:951–957
Abdel Aal A, Barakat M, Mohamed R (2008) Electrophoreted Zn–TiO2–ZnO nanocomposite coating films for photocatalytic degradation of 2-chlorophenol. Appl Surf Sci 254:4577–4583
Anderson C, Bard A (1995) An improved photocatalyst of TiO2/SiO2 prepared by a sol-gel synthesis. J Phys Chem 99:9882–9885
Anpo M, Tanahashi I, Kubokawa Y (1980) Photoluminescence and photoreduction of vanadium pentoxide supported on porous Vycor glass. J Phys Chem 84:3440–3443
Brinker C, Scherer G (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, New York
Chu D, Masuda Y, Ohji T, Kato K (2010) Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir 26:2811–2815
Chun H, Yizhong W, Hongxiao T (2001) Preparation and characterization of surface bond-conjugated TiO2/SiO2 and photocatalysis for azo dyes. Appl Catal B Environ 30:277–285
Fouad O, Ismail A, Zaki Z, Mohamed R (2006) Zinc oxide thin films prepared by thermal evaporation deposition and its photocatalytic activity. Appl Catal B Environ 62:144–149
Gao J, Luan X, Wang J, Wang B, Li K, Li Y, Kang P, Han G (2011) Preparation of Er3+:YAlO3/Fe-doped TiO2–ZnO and its application in photocatalytic degradation of dyes under solar light irradiation. Desalination 268:68–75
Height M, Pratsinis S, Mekasuwandumrong O, Praserthdam P (2006) Ag–ZnO catalysts for UV-photodegradation of methylene blue. Appl Catal B Environ 63:305–312
Inagaki M, Imai T, Yoshikawa T, Tryba B (2004) Photocatalytic activity of anatase powders for oxidation of methylene blue in water and diluted NO gas. Appl Catal B Environ 51:247–254
Ismail A, Ibrahim I, Mohamed R (2003) Sol–gel synthesis of vanadia–silica for photocatalytic degradation of cyanide. Appl Catal B Environ 45:161–166
Ismail A, Ibrahim I, Ahmed M, Mohamed R, El-Shall H (2004) Sol–gel synthesis of titania–silica photocatalyst for cyanide photodegradation. J Photochem Photobiol Sci A Chem 163:445–451
Jung K, Kang Y, Park S (1997) Photodegradation of trichloroethylene using nanometre-sized ZnO particles prepared by spray pyrolysis. J Mater Sci Lett 16:1848–1849
Klein L (1985) Sol–gel processing of silicates. Annu Rev Mater Sci 15:227–248
Li F, Huang X, Jiang Y, Liu L, Li Z (2009) Synthesis and characterization of ZnO/SiO2 core/shell nanocomposites and hollow SiO2 nanostructures. Mater Res Bull 44:437–441
Meng Z, Juan Z (2008) Wastewater treatment by photocatalytic oxidation of nano-ZnO. Glob Environ Pol Jpn 12:1–9
Minero C, Lucchiari M, Vione D, Maurino V (2005) Fe(III)-enhanced sonochemical degradation of methylene blue in aqueous solution. Environ Sci Technol 39:8936–8942
Movahedi M, Mahjoub A, Janitabar-Darzi S (2009) Photodegradation of congo red in aqueous solution on ZnO as an alternative catalyst to TiO2. J Iran Chem Soc 6:570–577
Qourzal S, Barka N, Tamimi M, Assabbane A, Nounah A, Ihlal A, Ait-Ichou Y (2009) Sol–gel synthesis of TiO2–SiO2 photocatalyst for β-naphthol photodegradation. Mater Sci Eng C 29:1616–1620
Ruetten S, Thomas J (2003) Photoinduced electron transfer at solid surfaces: the TiO2–SiO2 system. Photochem Photobiol Sci 2:1018–1022
Sakthivel S, Hidalgo M, Bahnemann D, Geissen S, Murugesan V, Vogelpohl A (2006) A fine route to tune the photocatalytic activity of TiO2. Appl Catal B Environ 63:31–40
Shen W, Li Z, Wang H, Liu Y, Guo Q, Zhang Y (2008) Photocatalytic degradation for methylene blue using zinc oxide prepared by codeposition and sol–gel methods. J Hazard Mater 152:172–175
Singhal A, Achary S, Tyagi A, Manna P, Yusuf S (2008) Colloidal Fe-doped ZnO nanocrystals: facile low temperature synthesis, characterization and properties. Mater Sci Eng B 153:47–52
Sivakumar T, Shanthi K, Newton Samuel T (2000) Photocatalysed decomposition of anthraquinone sulphonic acid (sodium salt) using ZnO. Bioprocess Eng 23:579–583
Walther K, Wokaun A, Handy B, Baiker A (1991) TiO2/SiO2 mixed oxide catalysts prepared by sol–gel techniques: characterization by solid state CP/MAS spectroscopy. J Non-Cryst Solids 134:47–57
Yu H, Wang S (2000) Effects of water content and pH on gel-derived TiO2–SiO2. J Non-Cryst Solids 261:260–267
Yu D, Cai R, Liu Z (2004) Studies on the photodegradation of Rhodamine dyes on nanometer-sized zinc oxide. Spectrochim Act Part A 60:1617–1624
Acknowledgments
The authors wish to express their sincere thanks to the King Abdulaziz City for Science and Technology (KACST), Saudi Arabia, for providing financial support (Grant number: P–S-10-0028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Mohamed, R.M., Baeissa, E.S., Mkhalid, I.A. et al. Optimization of preparation conditions of ZnO–SiO2 xerogel by sol–gel technique for photodegradation of methylene blue dye. Appl Nanosci 3, 57–63 (2013). https://doi.org/10.1007/s13204-012-0074-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-012-0074-z