Abstract
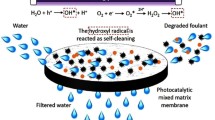
Immobilization of nano-scaled TiO2 onto polymeric ultrafiltration (UF) membrane offers desirable antifouling and self-cleaning properties to the membrane, which is practical in wastewater purification only if the mechanical strength and long-term self-cleaning durability are realized. This paper reported the surface roughness, mechanical properties, thermal stability, and recycling self-cleaning performance of the novel TiO2/PAA/PTFE UF membranes, which were coated via an innovative plasma-intensified coating strategy. Through careful characterizations, the enhanced engineering properties and the self-cleaning performance were correlated with the surface chemical composition and the creative coating technique. In the recycling photocatalytic self-cleaning tests in photodegradation of methylene blue (MB) solution, about 90 % MB photocatalytic capability of TiO2/PAA/PTFE composite membranes could be recovered with simple hydraulic cleaning combined with UV irradiation. The mechanical properties and thermal stability of TiO2/PAA/PTFE also satisfy the practical application in water and wastewater treatments, despite that the original engineering properties were slightly influenced by PAA grafting and TiO2 coating. The changed properties of the composite UF membrane relative to PTFE are reasonably attributed to the variation of the surface chemical species and chemical bonding, as well as the thickness and evenness of the surface functional layers.
Similar content being viewed by others
Introduction
Polytetrafluoroethylene (PTFE) is featured with relatively high thermal stability, excellent chemical inertness, low surface tension, and small coefficient of friction, which endow PTFE with an excellent performance in diverse applications including low friction films, seals, electronic and biomedical devices. In particular, porous PTFE membranes have found special importance in water treatment [1], separators of lithium-ion batteries [1, 2], pervaporation [3], and blood purification [4]. However, the presence of strong C–F bonding and water repellence makes the application of PTFE membranes in water treatment field less competitive because of the rather low water flux. Therefore, there is an increasing demand for hydrophilic modification of PTFE membranes to fulfill the requirements of wastewater treatment.
Generally, membranes are modified via five principal methods: (1) photo-initiated grafting and miscellaneous grafting; (2) plasma treatment; (3) physical coating/adsorption; (4) chemical reaction modification; (5) surface modification of membranes via nanoparticles impregnation [5]. The current trend is to coat or incorporate nano metal dioxide particles with the bulk membrane materials to enhance the performance of the membranes, such as hydrophilicity, permeate flux, photocatalytic activity, antifouling, water purification, and pollutant removal [6]. Titanium dioxide (TiO2) is one of the most applied nanoparticles due to its high stability, low cost, non-toxicity to environment and humans, chemical resistance, high photocatalytic activity, ability to mineralize organic pollutants and to kill bacteria [7, 8]. However, owing to the intrinsic non-polar linear molecular configuration of C and F atoms in PTFE, the surface modification of PTFE membranes via TiO2 nanoparticles faces great challenges in building strong adherence between TiO2 and PTFE. To tackle the challenges, at least two processes should be realized: (1) activating the intrinsic chemical inertia of PTFE via breaking the ultra-stable C–F bonding, (2) providing sufficient bonding sites to fix TiO2 tightly via surface functional groups [9–11].
Among the methods breaking C–F bonding on the surface of PTFE membranes, plasma technique has many advantages, because it avoids environmental contamination problems. Plasma assisted cross-linking and functional group attachment can also be surface-specified and leave little damage to mechanical properties of the bulk membranes [5]. It has been reported that N2, NH3, H2O, C2H2, and H2O/Ar plasmas effectively improve the surface hydrophilic properties by cross-linking or functionalization mechanisms [12, 13]. The Ar, O2 or Ar/O2 plasmas are used to improve surface adhesion of PTFE mainly by etching-induced surface activation and roughening effects [14]. But, plasma-generated radicals on the polymer surface would react rapidly once exposed to gaseous monomers [15]. Plasma-initiated graft polymerization might take good use of the short-lifetime surface radicals generated during the plasma activation and allow the growth of the graft macromolecular chain. Grafting density and length of the grafted chains can be controlled by tuning plasma parameters (power, pressure, sample disposition and so on) and polymerization conditions (monomer concentration, grafting time and so on). Acrylic acid is one of the most frequently applied monomers in membrane surface hydrophilic modification [16–18]. The negatively charged polyacrylic acid (PAA) layer could reduce the adsorption of negatively charged contaminants due to an enhanced electrostatic repulsive force between solutes and the modified membrane surfaces. Also, the carboxyl groups in PAA would, in turn, provide sufficient bonding sites for inorganic oxide nanoparticles [19]. This strategy was successfully applied in coating TiO2 on polyvinylidene difluoride (PVDF) membrane using TiO2 nanoparticles [20] or sol–gel [21], however, the mechanical stability and recycling application were not presented therein.
Recently, we developed a plasma-intensified coating process to fabricate TiO2/PAA/PTFE composite membrane [22], and the composite membrane exhibited excellent initial hydrophilicity, high ultrafiltration performance, antifouling ability as well as photocatalytic self-cleaning capability, which closely related to the property of TiO2 layer. However, the practical application of such membrane must fulfill the strict durability requirements to the engineering and mechanical properties of the TiO2 coating, which highly depends on its surface roughness and composition. In this paper, firstly, the physical and chemical natures of membrane surface were characterized by AFM and XPS. The mechanical property, the stability of photocatalytic self-cleaning in prolonged time and the thermal stability for the adoptability to strict application environment were further evaluated.
Experiments
Materials
Bare PTFE porous membranes manufactured by stretching process with mean pore diameter of 0.5 μm, with PET as the substrate and PTFE microfibers to form function surface, were both supplied by Valqua Shanghai Co., Ltd. (China). Ti(OBu)4, acrylic acid (AA), potassium persulfate, and bovine serum albumin (BSA; M w = 67,000 Da) were purchased from Sinopharm Chemical Reagent Co., Ltd (China). Analytical grade acetic acid, nitric acid, absolute ethanol, and ethylene glycol were obtained from Shanghai Lingfeng Chemical Reagent Co., Ltd (China). P25 was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. All reagents were used without further purification. Distilled water was used throughout the study.
Membrane modification procedures
The modification methodology was developed in our previous study [22], including the successive three-step process: plasma pretreatment of PTFE, graft polymerization of AA onto plasma-treated PTFE to form PAA/PTFE, and TiO2 self-assembly onto the PAA/PTFE to obtain the composite membranes. The mass-gaining rate of the PTFE membrane after introducing AA and TiO2 to the surface was calculated using the following Eq. (1):
where R is the mass-gaining rate, M t is the mass of the treated and dried membrane, and M 0 is the mass of the membrane that the treatment is based on.
Membrane characterization
Atomic force microscopy (AFM) was employed to analyze the surface morphology and roughness of the membranes using Nanoscope Multimode (Digital Instrument, USA). Approximately 1 cm2 of the as-prepared membranes were cut and glued on the glass substrate before AFM scanning (10 × 10 μm) under non-contact mode. A CAM110 contact angle-measuring device (Taiwan) was used to analyze contact angle and surface free energy. The surface chemical composition and functional groups of the membranes were investigated using Kratos AXIS Ultra DLD X-ray photoelectron spectroscope (Japan) and a Thermo Fisher Nicolet 6700 Fourier transform infrared spectrometer (USA), respectively. Mechanical property and thermal stability measurements were performed on an AGS-X universal testing machine (Shimadzu Corp., Japan) and PerkinElmer Pyris 1 thermogravimetric analysis (TGA) instrument, respectively.
Photocatalytic degradation of MB
Methylene blue (MB) was used as a model pollutant to evaluate the photodegradation ability of PTFE/PAA/TiO2 composite membranes under full-arc Xe-lamp irradiation. The composite membrane with certain area was tailored to unify the TiO2 loading of 10 mg on the membrane, and then immersed into 100-mL methylene blue (MB, 10 mg/L) aqueous solution in a beaker. The pH of the MB solution was adjusted to neutral (pH 7.0) using dilute sodium hydroxide (NaOH) and chloride acid (HCl). The beaker was positioned directly under full-arc irradiation generated by a 300-W Xe lamp (15 cm above the dishes) with a 400-nm cutoff filter as a light source. The MB solution and the TiO2/PAA/PTFE composite membrane were mixed on a magnetic stirring machine and remained in dark for an hour to establish a MB solution adsorption–desorption equilibrium on the membrane before light irradiation. During photocatalysis, 4-mL reaction solution was taken out every 15 min and filtered so as to measure the concentration change of MB using a UV–visible spectrophotometer (PerkinElmer, Lambda 650, USA). For comparison, the photodegradation of MB on bare PTFE membrane was also conducted using the same method.
Results and discussion
Surface topography and roughness
AFM was used to explore the topography and roughness of bare PTFE membranes and the composite membranes. The received 3D images are shown in Fig. 1, where the brightest area represents the highest point of the membrane surface, and the dark regions indicate the pores of the membranes. It can be clearly seen that the topology of the plasma-treated PTFE (Fig. 1b) is very similar to that of the original bare PTFE (Fig. 1a), revealing that the network structure with ridges and valleys on the surface of bare PTFE membrane remained after plasma treatment. It is reasonable as plasma treatment mainly modifies the surface chemical bonding, and the plasma-induced functional groups are prone to be quenched by environment moisture. However, the PAA graft and following TiO2 coating significantly varied the surface morphology and porous structure of PTFE. It can be seen from Fig. 1c that graft of PAA grafting layer not only reduced the roughness of the interknitting fibers but also narrowed some large pores among fibers, resulting in the change of membrane morphology from network to nodular structure. It could be speculated that the acrylic acid monomer adsorbed on the inner surface of plasma-treated PTFE membranes underwent grafting polymerization to form PAA coated onto PTFE which, in turn, brought to hydrophilicity of the PAA/PTFE membrane [22]. Such PAA coating provides sufficient bonding sites to fix TiO2 via coordination, because the abundant hydroxyl groups of PAA layer offer active coordinate sites to fix TiO2 at higher dispersion. This speculation is confirmed by the smoother morphology for TiO2/PAA/PTFE membrane presented in Fig. 1d.
The surface roughness of the membranes is quantitatively compared by the surface roughness parameters obtained from AFM analysis (Table 1). The surface roughness parameters to quantify the surface roughness include the mean roughness (R a), the root mean square of the height data associated with z axis (Rms), the mean height value of five local maxima and local minima (R 10z), surface area factor (S dr, the ratio between the actual and projected solid surface area, S dr = 1 for a smooth surface and >1 for a rough one), and roughness factor (r = 1 + S dr/100). The 2D roughness tendency can be described through the variation of R a, Rms, and R 10z, and the 3D roughness is reflected by the S dr and r parameters. The acquired roughness parameters are summarized in Table 1 and show similar roughness variation in terms of the stepwise modifications; therefore, for simplicity, we discuss one typical parameter, r, in detail, to conclude the modification influence on the membrane roughness.
Compared to bare PTFE membrane (roughness factor of 1.433), a rougher surface is introduced by plasma bombardment as indicated by the greater r of 1.635, suggesting a certain number of fiber cleavages arising from C–F bond break. The roughness increased greatly after PAA grafting although the open pores were covered by grafted PAA. This change should be the result of new polymer chains introduced onto the skeleton of PTFE. The roughness factor for TiO2/PAA/PTFE membrane decreased greatly to 1.275 compared with 2.132 for PAA/PTFE membrane, which may be attributed to the uniform distribution of TiO2 and thick TiO2 coating layer (mass gain rate for TiO2/PAA/PTFE is 22 % compared to the mass of PAA/PTFE membrane). It is worth mentioning that the reduction of average pore size (listed in Table 1) arising from coating also contributed to the smoother surface of TiO2/PAA/PTFE membranes.
Taking the change of wettability parameters (listed in Table 1) into account, it can be concluded that the modification process increased the wettability of PTFE membrane successfully with the surface free energy of 72.2 mJ/m2 and contact angle of 35° for the as-prepared TiO2/PAA/PTFE membrane. According to the relationship between roughness and wettability established by Wenzel, the increase of surface roughness will enhance the wettability caused by the chemistry of the surface [23]. In other words, for a hydrophilic material, the rougher the surface, the larger the surface free energy, the smaller the contact angle, and the stronger the wettability [24]. Therefore, it is possible in the future to further increase the wettability of TiO2/PAA/PTFE membrane by reducing the TiO2 coating thickness and avoiding TiO2 nanoparticle agglomerates.
Evolution of C and O species on composite membrane surface
X-ray photoelectron spectroscopy (XPS) was employed to characterize the surface of the bare and modified PTFE membranes for understanding their surface composition evolution induced by modification treatments. In the previous study [22], the successful loading of TiO2 on the modified PTFE UF membrane was evidenced by XPS. In the current work, the evolution of C species and O species is further demonstrated by the high-resolution C1 s and O1 s XPS spectra for all the samples and their corresponding deconvolution in Fig. 2a, b.
Besides the adventitious carbon at BE of 284.5 eV due to surface contamination, the main peak in the C1 s spectra of bare PTFE can be well deconvoluted into two components at BE of 292 and 291.58 eV, corresponding to CF2 and CF bands, respectively. After plasma pretreatment, the intensity of the CF2 and C–F bonds declined, while a new shoulder peak arose next to adventitious C1 s peak (285.9 eV), which is assigned to surface C–N, C–O, and C–OH bonds due to N2 plasma treatment and air exposure. A significant peak appeared at approximately 289 eV due to O–C=O bonds in PAA after PAA grafting. Distinct from O1 s XPS of PTFE, an additional significant and broad peak at 537 eV in the plasma-treated PTFE is associated with the surface oxidation species of PTFE with O–C=O groups [25] induced by plasma treatment and air exposure, which diminished in PAA/PTFE and TiO2/PAA/PTFE due to the coverage of PAA and TiO2. The O1 s intensity was further increased after PAA grafting and TiO2 coating onto the PAA/PTFE membrane due to the increased amount of O species relative to PAA and TiO2. The sharp O1 s and its wide shoulder peaks for TiO2/PAA/PTFE can be fitted reasonably into three peaks, corresponding to Ti–O (528.6 eV), C=O (533 eV), C–O and –OH groups.
The results suggested that the plasma treatment combined with air exposure led not only to the oxidation of PTFE, but also to the substantial surface defluoration, which corresponds to the reduction of CF2 peak area by FTIR analysis (as shown in Fig. 2c). Taking into account the decrease in the F/C ratio and the increase in the O/C ratio as shown in Fig. 2d, it can be concluded that the intrinsic C–F bonds on PTFE surface could be effectively broken, and the polar covalent bonds, such as C–C, C–O, C=O, were introduced by the as-proposed modification protocols, which resulted in the increase of the surface free energy and hydrophilicity of PTFE membrane (Table 1).
Mechanical property
The mechanical properties of bare PTFE membrane and PTFE-based composite membranes were examined. The results of tensile strength and elongation at break are shown in Table 2. After plasma treatment, the tensile strength and elongation were slightly decreased from 30.87 MPa and 28.15 % for bare PTFE to 30.08 MPa and 26.55 %, respectively. It demonstrates that treatment time and power used in plasma bombardment were well combined so that the break of C–F bonds by the plasma treatment (see AMF and XPS analyses) did not damage the mechanical property to some noticeable extent. Once grafted by PAA on the surface of plasma-pretreated PTFE membranes, the PAA/PTFE membrane gained reinforced mechanical strength with an increase of about 33–50 % for tensile strength and elongation, respectively. This might be benefited from covalent bonding between PAA and PTFE skeleton as well as the entanglements between polymer chains. However, after TiO2 coating, the tensile strength of TiO2/PAA/PTFE membrane was greatly deteriorated to 26.35 MPa, even lower than that of plasma-treated PTFE membrane. To explain the phenomenon, the formation process of TiO2 coating layer should be mentioned here. The plasma-intensified grafting of PAA layer could form coordination interactions with titanium precursor. The coordinated titanium precursor underwent hydrolysis and condensation reactions to generate TiO2 nanoparticles (NPs) on the surface of PAA/PTFE membranes. The coordination interaction favors the molecular-level dispersion of TiO2, leading to long-term stable residence and high mechanical strength. Nevertheless, the formation of chain bridge among neighboring particles at high particle loading would facilitate the undesirable aggregation of TiO2 nanoparticle and, therefore, retard the uniformity coating of TiO2, leading to unbalanced stress distribution on membrane matrix. The tensile strength of TiO2/PAA/PTFE membrane is within the safe range for membrane application in water and wastewater treatment, indicating that there is room to further optimize the loading and distribution of TiO2 NPs for improved overall performance.
Thermal stability
It is well known that the excellent thermal stability of PTFE is desirable in a wide range of applications due to its ultra-stable C–F bonds and the intrinsic chemical inertia. As demonstrated in XPS and AFM study, the C–F bonds of PTFE could be broken partially by plasma treatment, which definitely deteriorates the thermal stability of PTFE membrane to some degree depending on the duration and strength of plasma treatment. On the other hand, coating of TiO2 on the surfaces of PTFE membranes would increase the thermal stability of the modified membranes, since TiO2 is thermally stable over 1000 °C in air atmosphere. Therefore, it is uncertain if the established three-step modification protocol influenced the thermal stability of the as-prepared PTFE-based membranes, which may be evaluated using TGA. The obtained results are shown in Fig. 3, where four temperature regions are marked for the convenience of discussion.
As shown in TGA curves, the main decomposition regions of the bare PTFE membrane and plasma-treated PTFE membrane are located in region III and region IV. Region III occured between 410 and 550 °C, which might be attributed to PTFE matrix and PET substrate [26]. Region IV was up to 700 °C. The weight loss of plasma-treated PTFE membrane was a little bit less than bare PTFE membrane at the end of region III but about 15 % higher at the end of region IV. Taking the chemical reaction during plasma treatment and after air exposure into accont, the break of C–F bond by plasma bombardment might reduce the mass percentage of PTFE matrix decomposing in region III, while the generation of oxidation species of PTFE in air environment might increase the mass percentage of chemicals decomposed in region IV. Therefore, the TGA curves showed that the thermal stability of the bare PTFE membrane was damaged by plasma treatment.
For TGA curves of the PAA/PTFE membrane and TiO2/PAA/PTFE membrane, decomposition occured in all the four regions. Region I, before 150 °C, was attributed to the loss of absorbed water. Since the water uptake ability of TiO2/PAA/PTFE membrane was stronger than PAA/PTFE membrane, the weight loss of TiO2/PAA/PTFE membrane was higher in the region. Region II was between 200 and 400 °C, which was attributed to decomposition of PAA [27]. The weight loss of approximately 12 % in this region was well in line with the mass gain rate for PAA grafting. The less weight loss of TiO2/PAA/PTFE membrane than PAA/PTFE membrane in region II also suggested the successful incorporation of TiO2 into PAA. In Region III, there was a trend that the weight loss was decreased along the successive modification steps. It demonstrated that the weight percentage of PTFE matrix and PET substrate was reduced step by step. In other words, it verified the successful coating of PAA and TiO2. In region IV, temperature of weight loss region increased gradually for plasma-pretreated PTFE, PAA/PTFE, and TiO2/PAA/PTFE, which indicated the enhanced thermal stability. It can be explained that the covalent bonds formed by both PAA grafting on plasma-pretreated PTFE and TiO2 incorporation with PAA restricted the mobility of macromolecules and enhanced the energy needed for movement and breakage of polymeric chains. As expected, the weight loss stopped at 750 °C for TiO2/PAA/PTFE with the residue weight percentage of 22 % consistent with the mass gain rate for TiO2 coating.
It is worth noting that all the modification treatment may enhance the high temperature stability, while the PAA grafting exhibited the most serious impact on low-temperature thermal stability. However, in practice, the general water treatment temperature may not reach 150 °C, hence the decrease in low-temperature thermal stability would not hinder the application of TiO2-modified PTFE membrane in water treatment.
Photocatalytic stability
The photocatalytic stability of the TiO2/PAA/PTFE composite membrane was tested via monitoring the variation of MB equilibrium concentration along with full-arc light illumination in the 100-min recycling tests for five times. In each test, following 100-min photodegradation, the membrane was subject to the typical hydraulic washing regeneration, simply rinsing and washing the membrane in DI water for several times until the washing liquid is colorless. The acquired MB removal rate in each cycle is comparatively presented in Fig. 4a. It can be seen that almost 90 % MB was removed in the first test, while the MB degradation rate gradually declined to 20 % at the end of fifth cycle. The declined degradation rate may be due to the competitive surface adsorption of MB and incompletely decomposed intermediates, which was confirmed by the initial equilibrium adsorption rates before each recycling test. As shown in Fig. 4b, the MB adsorption rates in dark for the first two photocatalysis cycles were comparable and very high while remained stable in the following cycles. Meanwhile, the composite membrane turned to be blue after thoroughly washing by the end of the fifth photocatalysis test because of the adsorption of refractory photodegradation intermediates. This blue color of the used membrane became shallow once treated with 15-min UV irradiation, and the photocatalytic self-cleaning efficiency was almost fully recovered, as shown in the sixth plot in Fig. 4a. Therefore, the loss of photodegradation ability of TiO2/PAA/PTFE membrane can be reasonably attributed to the competitive photodegradation between MB and its intermediates firmly adsorbed on the membrane in the previous photocatalysis cycles. The result not only verified the stability of photodegradation ability endowed by TiO2 functional layer, but also implied that the hydraulic cleaning combined with UV irradiation is an efficient strategy for regeneration of fouled TiO2/PAA/PTFE membranes in practice.
Conclusion
The surface roughness, mechanical strength, and self-cleaning capability of the TiO2/PAA/PTFE composite membrane fabricated with plasma-intensified coating process, were carefully investigated to evaluate its potential for practical wastewater ultrafiltration. The influences of the fabrication process on the membrane’s surface chemistry and engineering properties were also studied.
Plasma treatment may lead to surface defluoration of PTFE, and the following air exposure contributes to initiating and stabilizing the formation of polar species on PTFE, which facilitates PAA grafting and sequential tight coating of TiO2. The recycling photocatalytic self-cleaning tests revealed that the TiO2/PAA/PTFE composite membranes are very stable, which is due to the tight bonding between the TiO2, PAA, and PTFE as evidenced by XPS and mechanical characterizations. The mechanical properties and thermal stability of TiO2/PAA/PTFE satisfy the application in wastewater treatment, despite that the original engineering properties were slightly influenced by PAA grafting and TiO2 coating. The enhanced properties of the composite membrane relative to PTFE ultrafiltration membranes are related to the surface chemical species and chemical bonding, as well as the thickness and evenness of the surface functional layers.
References
Bamperng S, Suwannachart T, Atchariyawut S, Jiraratananon R (2010) Ozonation of dye wastewater by membrane contactor using PVDF and PTFE membranes. Sep Purif Technol 72:186–193. doi:10.1016/j.seppur.2010.02.006
Amanchukwu CV, Harding JR, Shao-Horn Y, Hammond PT (2015) Understanding the chemical stability of polymers for lithium-air batteries. Chem Mater 27:550–561. doi:10.1021/cm5040003
Yu C-H, Kusumawardhana I, Lai J-Y, Liu Y-L (2009) PTFE/polyamide thin-film composite membranes using PTFE films modified with ethylene diamine polymer and interfacial polymerization: preparation and pervaporation application. J Colloid Interface Sci 336:260–267. doi:10.1016/j.jcis.2009.03.052
Roy-Chaudhury P, Kelly BS, Melhem M et al (2005) Novel therapies for hemodialysis vascular access dysfunction: fact or fiction! Blood Purif 23:29–35. doi:10.1159/000082008
Kochkodan V, Johnson DJ, Hilal N (2014) Polymeric membranes: surface modification for minimizing (bio) colloidal fouling. Adv Colloid Interface Sci 206:116–140. doi:10.1016/j.cis.2013.05.005
Ng LY, Mohammad AW, Leo CP, Hilal N (2013) Polymeric membranes incorporated with metal/metal oxide nanoparticles: a comprehensive review. Desalination 308:15–33. doi:10.1016/j.desal.2010.11.033
Singh S, Mahalingam H, Singh PK (2013) Polymer-supported titanium dioxide photocatalysts for environmental remediation: a review. Appl Catal A Gen 462:178–195. doi:10.1016/j.apcata.2013.04.039
Liao Y, Que W, Zhong P et al. (2011) A facile method to crystallize amorphous anodized TiO2 nanotubes at low temperature. ACS Appl Mater Interfaces 3:2800–2804. doi:10.1021/am200685s
Razmjou A, Mansouri J, Chen V et al. (2011) Titania nanocomposite polyethersulfone ultrafiltration membranes fabricated using a low temperature hydrothermal coating process. J Membr Sci 380:98–113. doi:10.1016/j.memsci.2011.06.035
Shan AY, Ghazi TIM, Rashid SA (2010) Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: a review. Appl Catal A Gen 389:1–8. doi:10.1016/j.apcata.2010.08.053
Fischer K, Grimm M, Meyers J et al. (2015) Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J Membr Sci 478:49–57. doi:10.1016/j.memsci.2015.01.009
Vandencasteele N, Merche D, Reniers F (2006) XPS and contact angle study of N2 and O2 plasma-modified PTFE, PVDF and PVF surfaces. Surf Interface Anal 38:526–530. doi:10.1002/sia.2255
Abourayana HM, Dowling DP (2015) Plasma processing for tailoring the surface properties of polymers. In: Mahmood A (ed) Surface energy. InTech, Rijeka, Croatia
Carbone E, Verhoeven M, Keuning W (2013) PTFE treatment by remote atmospheric Ar/O2 plasmas: a simple reaction scheme model proposal. J Phy Conf Ser 715(1):1–6. doi:10.1088/1742-6596/715/1/012011
Chien HH, Ma KJ, Kuo CH, Huang SW (2013) Effects of plasma power and reaction gases on the surface properties of ePTFE materials during a plasma modification process. Surf Coat Technol 228:S477–S481. doi:10.1016/j.surfcoat.2012.05.014
Fang B, Ling Q, Zhao W et al (2009) Modification of polyethersulfone membrane by grafting bovine serum albumin on the surface of polyethersulfone/poly(acrylonitrile-co-acrylic acid) blended membrane. J Membr Sci 329:46–55. doi:10.1016/j.memsci.2008.12.008
Yu H, Xu Z, Yang Q et al (2006) Improvement of the antifouling characteristics for polypropylene microporous membranes by the sequential photoinduced graft polymerization of acrylic acid. J Membr Sci 281:658–665. doi:10.1016/j.memsci.2006.04.036
Xu Z, Wang J, Shen L et al (2002) Microporous polypropylene hollow fiber membrane: part I. Surface modification by the graft polymerization of acrylic acid. J Membr Sci 196(2):221–229
Madaeni SS, Zinadini S, Vatanpour V (2011) A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J Membr Sci 380:155–162. doi:10.1016/j.memsci.2011.07.006
You S-J, Semblante GU, Lu S-C et al (2012) Evaluation of the antifouling and photocatalytic properties of poly (vinylidene fluoride) plasma-grafted poly (acrylic acid) membrane with self-assembled TiO2. J Hazard Mater 237:10–19. doi:10.1016/j.jhazmat.2012.07.071
Zhang F, Zhang W, Yu Y et al (2013) Sol–gel preparation of PAA-g-PVDF/TiO2 nanocomposite hollow fiber membranes with extremely high water flux and improved antifouling property. J Membr Sci 432:25–32. doi:10.1016/j.memsci.2012.12.041
Qian Y, Chi L, Zhou W et al (2016) Fabrication of TiO2-modified polytetrafluoroethylene ultrafiltration membranes via plasma-enhanced surface graft pretreatment. Appl Surf Sci 360:749–757. doi:10.1016/j.apsusc.2015.11.059
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28:988–994. doi:10.1021/ie50320a024
Wei X, Guo-Xin X, Quan W et al (2009) The surface energy and nano-adhesion behavior of some micro-component material in MEMS. Acta Phys Sin 58:2518–2522
Clark DT, Feast WJ, Tweedale PJ (1980) ESCA applied to polymers. XXVI. Investigation of a series of aliphatic, aromatic, and fluorine-containing polycarbonates. J Polym Sci Polym Chem Ed 18(6):1651–1664
Costache MC, Heidecker MJ, Manias E, Wilkie CA (2006) Preparation and characterization of poly(ethylene terephthalate)/clay nanocomposites by melt blending using thermally stable surfactants. Polym Adv Technol 17:764–771. doi:10.1002/pat.752
Moharram MA, Allam MA (2007) Study of the interaction of poly (acrylic acid) and poly (acrylic acid-poly acrylamide) complex with bone powders and hydroxyapatite by using TGA and DSC. J Appl Polym Sci 105:3220–3227. doi:10.1002/app.26267
Acknowledgments
We acknowledge the financial support from Shanghai Jiao Tong University, China, Shanghai Lee’s FUDA Electromechanical Co.Ltd., China and the Newton Research Collaboration Award from Royal Academy of Engineering, UK (Reference: NRCP/1415/261). We also thank researchers in R&D Department in Shanghai VALQUA Company Limited for their kind supply of bare hydrophobic PTFE membranes and commercial hydrophilic PTFE membranes.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chi, L., Qian, Y., Zhang, B. et al. Surface engineering and self-cleaning properties of the novel TiO2/PAA/PTFE ultrafiltration membranes. Appl Petrochem Res 6, 225–233 (2016). https://doi.org/10.1007/s13203-016-0158-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-016-0158-x