Abstract
The scope of the present study is to describe the cracking behavior of hydrocarbons and the reduction of sulfur in gasoline in fluid catalytic cracking (FCC) process using Zn–Mg–Al additives with varying the Mg/Al molar ratios. Experiments have been carried out on a micro-activity-test (MAT) reactor using high-sulfur vacuum gas oil (VGO) feed and zinc impregnated Mg–Al spinels as additive and the commercial cracking catalyst. It was found that Zn–Mg–Al additives exhibited enhanced Lewis acidity compared with the corresponding Zn-free Mg–Al spinels. The MAT results indicated that the addition of additives reduced the yields of liquid petroleum gas and coke at low Mg contents but increased the coke yield at high Mg contents. Overall, the additives improved the yields of gasoline and diesel. It has also been shown that the rich Lewis acidity had a positive effect on the conversion of aromatic sulfur species of gasoline and the maximum reduction of gasoline sulfur was achieved with Zn/Mg4.0Al2O3 due to the synergistic effect of basicity and Lewis acidity.
Similar content being viewed by others

Introduction
Environmental protection nowadays has been a general consensus worldwide and the better quality of motor fuels is required in the legislation of many countries [1, 2]. In this sense, sulfur contents in gasoline and diesel are expected to be reduced toward 10 and 50 ppm since the year 2010, respectively [3, 4]. In the case of gasoline pool, nearly 90 % of sulfur content comes from fluid catalytic cracking (FCC) gasoline in China and about 33 % in USA. Thus, an important effort of refineries is devoted to effectively reduce the sulfur coming from FCC unit by already existing technologies or developing more efficient and economical methods.
Hydrotreating of FCC feedstock and hydrodesulfurization (HDS) of FCC gasoline have been the commonly used and most effective processes for removing sulfur compounds [5]. However, they are limited by the high capital investment and operation costs, particularly the loss of octane number of gasoline in HDS process. Catalytic technologies, named as sulfur reducing additives, have been developed and should be the most economical and easiest implement among many new approaches, such as adsorption, oxidation, and extraction reactions for refineries [2, 6–8].
The additives, typically the Zn, Zr, Mn, etc. doped metal oxides, can reduce the sulfur content in FCC gasoline by 10–30 % with less than 10 wt% of dosage in base FCC catalysts [9, 10]. Generally, alumina supported zinc oxide (ZnO/Al2O3) are the commonly used components of additives and possess enhanced Lewis acidity which contributes to the adsorption and conversion of sulfur compounds. This mechanism has been more widely adopted [11]. However, recent research found that additives with mixed metal oxides as supporter, expressed as Mg(Al)O with the varying amounts of alkaline MgO, were also valid for reducing sulfur content of FCC gasoline [10, 12, 13]. Myrstad et al. [12] found Zn/Mg(Al)O additive had an inferior effect in reducing the sulfur content of naphtha to Zn/Al2O3 in the same experimental conditions. In contrast, the former was found to be more effective for reducing the sulfur of gasoline as reported by Andersson et al. [10]. However, the composition of additives was not given in both reports. Vargas-Tah et al. [13]. proposed that the incremental substitution of Zn by Mg on Zn–Mg–Al additives reduced the Lewis acidity of the materials. But the correlation between acidity and sulfur reducing performance of FCC gasoline were not provided. In addition, the blends of additive and base catalyst usually caused the decrease of the conversion of feedstock and the increase of coke formation to a certain degree because of the dilution effect of base catalysts and the enhanced Lewis acidity on additives.
In this work, we studied the Lewis acidity of Zn–Mg–Al additives with the varying Mg contents and its effects on the performance of catalytic reactions of hydrocarbons and sulfur reduction of FCC gasoline.
Experimental section
Catalyst preparation
Mg–Al spinels were firstly prepared by the hydrothermal treatment and post-calcination method. The mixed aqueous solution of Mg(NO3)2 and Al(NO3)3 with different Mg/Al molar ratios (0.25, 0.5, 1.0, and 2.0) and glucose as template was titrated quickly with NaOH solution to completely precipitate the metal ions. The suspensions then were hydrothermally treated in a stainless steel vessel at 100 °C for 24 h and followed by filtrating, drying, and heating at 550 °C for 4 h. Thereafter, the prepared Mg–Al spinels were impregnated with Zn(NO3)2 aqueous solution of 0.8 mol/L at room temperature for 5 h. The catalysts were finally prepared with 10 wt% of ZnO on Zn–Mg–Al after calcination at 700 °C for 3 h.
Characterization
The crystalline phase of the synthesized samples was determined by X-ray powder diffraction (XRD) patterns with a Bruker Axs diffractometer (Germany) using Cu-Kα radiation generated at 40 kV and 40 mA, scanning range from 5 to 80° at a speed of 0.01o/s. N2 sorption measurements at −196 °C were carried out in a Micromeritics TRISTAR 3000 analyzer. The samples were previously outgassed at 300 °C for 3 h. Specific surface area (SBET) was calculated by the BET method using experimental points at a relative pressure of P/P0 = 0.05–0.25. The pore-size distribution was derived from the desorption branch, using the BJH method [14]. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 6,700 spectrometer with a wide-band mercury–cadmium–telluride (MCT) liquid-nitrogen-cooled detector and a KBr beam splitter. The spectra of the samples were recorded by accumulating 64 scans at 4 cm−1 resolution in the spectral range of 500–4,000 cm−1. The samples were firstly dehydrated at 400 °C for 2 h under vacuum pressure and then cooled to the room temperature. Then the pyridine vapor was introduced for equilibrium adsorption and the system then was treated at constant temperature of 120 °C and <2 × 10−3 Pa for 2 h allowing the removal of physically adsorbed pyridine.
Catalytic evaluation
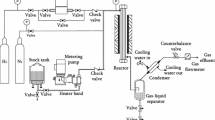
Catalytic activity tests of catalyst and additive were performed in an automated bench-scale micro-activity test (MAT) unit. The catalytic reactions occurred at 500 °C for 75 s in a tubular stainless steel reactor with an inner diameter of 13 mm and length of 180 mm and 1.048 g of VGO feedstock (properties are shown in Table 1). Each additive was blended with the industrial equilibrium FCC catalyst labeled LVR-60R (properties were shown in Table 1) with a mass ratio of 1:9. To change the conversion of VGO, the catalyst-to-oil (CTO) ratio in the experiments was varied from 3 to 6 by changing the amount of catalyst usage.
The resulting cracking gases were collected and analyzed by a Varian 3800 gas chromatograph (GC) equipped with two thermal conductivity detectors (TCD) and a flame ionization detector (FID). The liquid products were weighed and analyzed by simulated distillation on a Varian 3800 GC according to the ASTM D2887 method. So the mass percentage of gasoline (IBP-204 °C), diesel (204–350 °C), and slurry (>350 °C) were quantified. The Elemental Analyzer (Elementar Vario El III) was used for measuring the weight of coke deposited on the spent catalyst by analyzing the CO2 and CO quantities after combustion. The conversion of VGO was defined as the weight percentage of feedstock converted to dry gas, liquid petroleum gas (LPG), gasoline, diesel, and coke. The Elemental Analyzer was also used for measuring the weight of total sulfur in liquid products. The sulfur mass distribution in liquid products was analyzed by a gas chromatograph (GC-450) with a pulsed flame photometric detector (PFPD) for the detection of sulfur-containing compounds.
Results and discussion
The XRD patterns of the prepared Mg–Al spinels are observed in Fig. 1. The Mg-free sample had pure crystalline phase of the reflections of gamma alumina (ICDD, PDF 01-075-0921). With increasing Mg amounts, the γ-Al2O3-type crystalline phase was gradually transformed into the MgAl2O4 spinel phase at Mg/Al molar ratio of 0.5 and into a solid solution with overlapped characteristic peaks of Mg(Al)O periclase-type and MgAl2O4 spinel-type phases at a Mg/Al molar ratio of 2.0 [15, 16].
After impregnation of Zn, the ZnAl2O4 phase (ICDD, PDF 01-077-0732) was observed obviously which shielded the reflections of γ-Al2O3 crystalline phase although only 10 wt% of zinc oxide doping on γ-Al2O3 supporter (Fig. 2). It was ascribed to the similar 2θ positions of the reflections of ZnAl2O4 and γ-Al2O3. At the Mg/Al molar ratio of 0.5, the reflection intensities of ZnAl2O4 increased, however, it decreased accompanied with the appearance of another crystalline phase of ZnO (ICDD, PDF 01-070-2551) when the Mg/Al molar ratio was up to 2.0. In view of the excess Mg rendered the Al atoms existing in the form of MgAl2O4, the ZnO was easily separated out even at a low dosage.
To give an insight into the textural properties of additives, N2 adsorption and desorption isotherms and pore-size distribution for the samples are shown in Fig. 3. All prepared samples had type IV isotherms with pronounced H2 hysteresis loops (Fig. 3a), which were the characteristics of many mesoporous materials [17]. Taking Zn/Al2O3 sample for example, the N2 adsorption jump at the relative pressures of 0.4–0.8 was attributed to the capillary condensation in the mesopores [18]. The BJH pore-size distribution (Fig. 3b) demonstrated that all samples except Zn/Mg1.0Al2O3 exhibited the narrow pore-size distribution at about 3–7 nm.
For all samples, the specific surface areas (Table 2) decreased gradually from 199 to 128 m2 g−1 at first upon increasing the Mg/Al molar ratio from 0 to 0.5 and increased gradually with the increase of Mg/Al molar ratio from 0.5 to 2.0. However, the pore volume and average pore width demonstrated the reverse trends and their values firstly increased and then decreased with the increase of Mg/Al molar ratio. The biggest pore size might be attributed to the good atomic compatibility of MgAl2O4 supporter.
The acidity properties of all samples were determined by pyridine FT-IR spectra (Fig. 4). The pyridine IR spectra showed that all samples had only the Lewis acidic sites (LAS) with the characteristic band at ~1,445 cm−1 [19]. In addition, the associated shoulder band at higher wave number of 1,450 cm−1 was assigned to the LAS with stronger acidity strength [20, 21]. The results indicated that the amounts of LAS for Mg–Al spinels decreased with the increase of Mg/Al molar ratio. Although the content of alkaline MgO increased, the LAS amounts decreased firstly and then increased slightly for Zn–Mg–Al additives with the minimum acidity amount for Zn/Mg1.0Al2O3 (Table 2). It is noteworthy that both Mg2+ and Al3+ can be the LAS; therefore, at low Mg content, the LAS could be attributed to Al3+ sites while at high Mg content it should be attributed to Mg2+ sites [22]. The MgAl2O4 spinel phase at the Mg/Al molar ratio of 0.5 possessed the lowest amount of LAS because of the good atomic compatibility. Additionally, all Zn–Mg–Al additives had higher acidity amounts than the corresponding Mg–Al spinels, indicating that the LAS on Mg–Al spinels were enhanced so that the pyridine was easier to be adsorbed on them. More importantly, the Lewis acidity strength was also enhanced with the appearance of a shoulder band at 1,450 cm−1.
MAT conversion versus CTO ratio for base FCC catalyst (Ecat) and its blends with additive are shown in Fig. 5. The CTO ratio represents the average activity of catalysts that contact oil vapor. Therefore, the higher CTO ratio means the improvement of the contact opportunities between the active centers of catalysts and the hydrocarbon molecules. Generally, a higher CTO ratios are needed for blends with additive to achieve the same conversion compared with Ecat alone. It can be attributed to the dilution effect of additives which possess a much lower cracking activity for hydrocarbons compared with Ecat. However, the conversion of mixture with Zn/Al2O3 was slightly higher than that of Ecat at the same CTO ratio as reported previously [2]. It might be attributed to its highest acidity amount on all additives as described in this work (Table 2). It is noteworthy that Zn/Mg4.0Al2O3 had a lowest MAT conversion at the same CTO ratio although it had a relatively higher Lewis acidity than Zn/Mg1.0Al2O3 (Table 2). It might be attributed to the increased basicity of additive with the increase of alkaline MgO. Therefore, the catalytic activity of hydrocarbons associated with both the acidity and the basicity of additives [12, 23].
Figure. 6 shows the product yields of LPG, gasoline, diesel, and coke as a function of MAT conversion for Ecat without and with additive. The yields of LPG and coke formation increased with increasing the MAT conversion. Specifically, LPG yields were lower at conversion levels less than 78 % for additive-added catalysts compared with Ecat alone. However, it increased rapidly with increasing conversion for additive-added catalysts. In contrast, gasoline yield increased with increasing conversion for Ecat alone and it was lower than that with additive addition at the same conversion less than 80 %. At the same conversion for all blends with additives except Zn/Al2O3, diesel yields were a slightly higher than that for Ecat alone while the coke yields for all blends with additives except Zn/Al2O3 were slightly higher than that for Ecat alone. The highest coke yield of Zn/Mg4.0Al2O3 at the same conversion demonstrated that basic sites on it led to higher coke formation in spite of the existence of LAS.
Table 3 shows the MAT reaction data obtained for Ecat alone and its blend with additive at a constant VGO conversion of 77 wt%. Compared with Ecat alone, the CTO ratio decreased firstly and then increased with the increase of Mg/Al molar ratio to achieve the same conversion level. The LPG yield decreased firstly with the increase of Mg/Al molar ratios from 0 to 0.5 and increased thereafter from 0.5 to 2.0, but a reverse trend was observed for gasoline yield. However, with the increase of Mg/Al molar ratio diesel yield showed an upward tendency, but slurry yield showed a downward tendency. Because gasoline was typically susceptible to secondary reaction and undergoes over-cracking to produce LPG [8, 24], therefore, the Lewis acidity of Zn/Al2O3 additive enhanced the pre-cracking of slurry to form gasoline and diesel but reduced the secondary cracking of gasoline to form LPG [25].
Here, a coefficient of the hydrogen transfer parameter (CHT) was proposed to quantitatively analyze the degree of the hydrogen transfer reaction as reported in a few open literatures in which the CHT was defined as the ratio of the weight percentages between paraffin and olefin in LPG [3, 26]. Higher CHT indicated lower secondary cracking activity of liquid products especially gasoline and gasoline yields. Although the LAS amount was reduced after Mg doping when Mg/Al molar ratio was less than 1.0, the pre-cracking of VGO was enhanced due to the decreased activity of nonselective hydrogen transfer reactions which was reflected in the low yield of coke. In addition, the basic sites of MgO exhibited high hydrogen capacity that contributed to the adsorption and desorption of hydrogen [12, 27]. However, the Zn/Mg0.5Al2O3 had a higher CHT of 0.74 than that of Zn/Mg1.0Al2O3 (CHT = 0.62) although the latter had higher Mg content. Therefore, the analysis of comparative results of Zn/Mg0.5Al2O3 and Zn/Mg1.0Al2O3 indicated that only combining with the Lewis acidity could the high activity of hydrogen transfer be achieved [2, 11]. However, the excess Mg on additives suppressed the secondary cracking of gasoline and diesel when Mg/Al molar ratio was more than 1.0. Hence, the higher CTO ratio was needed to achieve the same conversion level that led to higher coke yield and yields of gasoline and diesel.
The distribution of sulfur species in FCC gasoline cut obtained at the VGO conversion of 77 % for Ecat alone and its blend with additive are displayed in Table 4. The sulfur reducing abilities was determined by the differences of sulfur contents in gasoline cuts between Ecat alone and additive-added blends. The sulfur contents in gasoline were drastically reduced by the blends adding with additives except Zn/Mg1.0Al2O3. The reduction efficiency of additive decreased firstly and increased with the increase of Mg/Al molar ratio and Zn/Mg4.0Al2O3 exhibited the highest efficiency of sulfur reduction to 21.66 %. Specifically, Zn/Al2O3 additive possessed the lower contents of thioethers, part of aromatic sulfur-containing compounds such as thiophene (Th)/tetrahydrothiophene (THT), and some unknown aliphatic sulfur species compared with Ecat. In contrast, Zn/Mg4.0Al2O3 additive reduced the content of aliphatic sulfur species instead of aromatic sulfur species. However, the contents of all sulfur species except some unknown sulfur species increased by Zn/Mg1.0Al2O3 additive, resulting in the increase of sulfur content of gasoline.
The detailed mechanisms for the sulfur removal reactions are not completely known yet, however, it is widely recognized that the LAS are the active sites for adsorbing sulfur-containing compounds onto the additive and/or base FCC catalyst and then cracking them at least the aliphatic sulfur species to form H2S [11]. An abundant of results in the open literatures suggested that there were two different reaction sequences accounting for the reduction of sulfur in FCC gasoline [28, 29]. The aromatic sulfur species were saturated by hydrogen transfer reactions and thereafter cracked to form H2S into gas phase, or the sulfur species could be transformed into heavier sulfur species which existed in other distillates out of gasoline boiling ranges. The results of sulfur distribution by Zn/Al2O3 additive in this work agreed well with the above conclusion. However, Zn/Mg4.0Al2O3 additive had an increased content of aromatic sulfur species but a decreased content of aliphatic sulfur species compared with Zn/Al2O3 additive. In combination with the Mg/Al molar ratio and Lewis acidity (Table 2), it was not hard to find that the reduced Lewis acidity of Zn/Mg4.0Al2O3 additive was not effective for adsorbing and cracking aromatic sulfur species. However, the rich basic sites were effective for absorbing aliphatic sulfur compounds especially the mercaptans which had a nature of acid and providing enough hydrogen to crack them [11, 30]. Therefore, the reduction of sulfur species in gasoline using Zn–Mg–Al type additive was attributed to the synergistic effect of LAS and basic sites on the additive [11, 12, 27].
Conclusion
Through this work, it has been shown that Zn–Mg–Al additives with the varying Mg contents and Lewis acidity made by impregnating Zn on Mg–Al spinels were able to give a significant impact on the conversion of VGO feed, coke formation, and sulfur reduction of FCC gasoline in the MAT experiments when blending with base FCC catalyst. The results have shown that the additive exhibited a positive effect on the conversion of VGO into gasoline and diesel, but gave unwanted decrease of the production of LPG. The excess Mg on additives gave unfavorable increase of coke formation. Additionally, it has been shown that the LAS played a key role in reducing the sulfur content of FCC gasoline following the similar ways as previously reported; however, the addition of excess Mg had a better effect on sulfur reduction particularly on aliphatic sulfur species due to the synergistic effect of basic sites and LAS on additives.
References
Wang R, Wan J, Li Y, Sun H (2014) An insight into effect of methanol on catalytic behavior of Amberlyst 35 resins for alkylation desulfurization of fluid catalytic cracking gasoline. Fuel 115:609–617
Quintana-Solórzano R, Valente JS, Hernández-Beltrán FJ, Castillo-Araiza CO (2008) Zinc-aluminates for an in situ sulfur reduction in cracked gasoline. Appl Catal B Environ 81:1–13
Wen Y, Wang G, Xu C, Gao J (2012) Study on in situ sulfur removal from gasoline in fluid catalytic cracking process. Energy Fuel 26:3201–3211
Song H, Wan X, Dai M, Zhang J, Li F, Song H (2013) Deep desulfurization of model gasoline by selective adsorption over Cu-Ce bimetal ion-exchanged Y zeolite. Fuel Process Technol 116:52–62
Bahzad D, Al-Fadhli J, Al-Dhafeeri A, Abdal A (2010) Assessment of selected apparent kinetic parameters of the HDM and HDS reactions of two Kuwaiti residual oils, using two types of commercial ARDS catalysts. Energy Fuel 24:1495–1501
Shu C, Sun T, Zhang H, Jia J, Lou Z (2014) A novel process for gasoline desulfurization based on extraction with ionic liquids and reduction by sodium borohydride. Fuel 121:72–78
Lü H, Deng C, Ren W, Yang X (2014) Oxidative desulfurization of model diesel using [(C4H9)4N]6Mo7O24 as a catalyst in ionic liquids. Fuel Process Technol 119:87–91
Quintana-Solórzano R, Rodríguez-Hernández A, García-De-León R (2008) Study of the performance of catalysts for catalytic cracking by applying a lump-based kinetic model. Ind Eng Chem Res 48:1163–1171
Siddiqui MaB, Ahmed S, Aitani a M, Dean CF (2006) Sulfur reduction in FCC gasoline using catalyst additives. Appl Catal A-Gen 303:116–120
Andersson POF, Pirjamali M, Järås SG, Boutonnet-Kizling M (1999) Cracking catalyst additives for sulfur removal from FCC gasoline. Catal Today 53:565–573
Can F, Travert A, Ruaux V, Gilson JP, Maugé F, Hu R, Wormsbecher RF (2007) FCC gasoline sulfur reduction additives: mechanism and active sites. J Catal 249:79–92
Myrstad T, Engan H, Seljestokken B, Rytter E (1999) Sulphur reduction of fluid catalytic cracking (FCC) naphtha by an in situ Zn/Mg(Al)O FCC additive. Appl Catal A-Gen 187:207–212
Vargas-Tah a A, García RC, Archila LFP, Solis JR, López a J G (2005) A study on sulfur reduction in FCC gasoline using Zn–Mg–Al spinels. Catal Today 107–108:713–718
Brunauer S, Emmett PH, Teller E (1938) Adsorption of Gases in Multimolecular Layers. J Am Chem Soc 60:309–319
Yoo JS, Bhattacharyya a A, Radlowski CA (1991) De-SOx catalyst: an XRD study of magnesium aluminate spinel and its solid solutions. Ind Eng Chem Res 30:1444–1448
Polato CMS, Henriques CA, Neto a A, Monteiro JLF (2005) Synthesis, characterization and evaluation of CeO2/Mg, Al-mixed oxides as catalysts for SOx removal. J Mol Catal A Chem 241:184–193
Zou L, Li F, Xiang X, Evans DG, Duan X (2006) Self-generated template pathway to high-surface-area zinc aluminate spinel with mesopore network from a single-source inorganic precursor. Chem Mater 18:5852–5859
Gregg SJ, Sing KSW (1983) Adsorption, surface area, and porosity, 2nd edn. Academic Press, London
Emeis CA (1993) Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J Catal 141:347–354
Tamura M, Shimizu K-I, Satsuma A (2012) Comprehensive IR study on acid/base properties of metal oxides. Appl Catal A- Gen 433–434:135–145
Matsunaga Y, Yamazaki H, Yokoi T, Tatsumi T, Kondo JN (2013) IR characterization of homogeneously mixed silica-alumina samples and dealuminated Y zeolites by using pyridine, CO, and propene probe molecules. J Phys Chem C 117:14043–14050
Busca G (2010) Bases and basic materials in chemical and environmental processes. Liquid versus solid basicity. Chem Rev 110:2217–2249
Xu X, Ran X, Cui Q, Li C, Shan H (2010) ZSM-5- and MgAl2O4-based bifunctional additives for enhancing the production of propene and removal of SO2 in the fluid catalytic cracking (FCC) process. Energy Fuel 24:3754–3759
Cumming KA, Wojciechowski BW (1996) Hydrogen transfer, coke formation, and catalyst decay and their role in the chain mechanism of catalytic cracking. Catal Rev 38:101–157
Meisel SL, Wojciechowski BW and Corma A (1987) Catalytic cracking: catalysis, chemistry, and kinetics. AIChE J 33:1581–1584
Myrstad T, Seljestokken B, Engan H, Rytter E (2000) Effect of nickel and vanadium on sulphur reduction of FCC naphtha. Appl Catal A-Gen 192:299–305
Barthomeuf D, Mallmann a D, P.J. Grobet W J M E F V, and Schulz-Ekloff G (1988) Basicity and electronegativity of zeolites Stud. Surf. Sci. Catal, Volume 37, pp. 365-374
Corma A, MartíNez C, Ketley G, Blair G (2001) On the mechanism of sulfur removal during catalytic cracking. Appl Catal A-Gen 208:135–152
Leflaive P, Lemberton JL, Pérot G, Mirgain C, Carriat JY, Colin JM (2002) On the origin of sulfur impurities in fluid catalytic cracking gasoline-reactivity of thiophene derivatives and of their possible precursors under FCC conditions. Appl Catal A-Gen 227:201–215
Potapenko OV, Doronin VP, Sorokina TP, Talsi VP, Likholobov VA (2012) Transformations of thiophene compounds under catalytic cracking conditions. Appl Catal B-Environ 117–118:177–184
Acknowledgments
This work was financially supported by the King Abdul Aziz City for Science and Technology (Project No. 649-32) and the Joint Funds of the National Natural Science Foundation of China and China National Petroleum Corporation (Grant No. U1362202).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Feng, R., Al-Megren, H., Li, X. et al. The effects of magnesium of Zn–Mg–Al additives on catalytic cracking of VGO and in situ reduction of sulfur in gasoline. Appl Petrochem Res 4, 329–336 (2014). https://doi.org/10.1007/s13203-014-0068-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-014-0068-8