Abstract
In this study, the degradation of crystal violet (CV) was investigated by different oxidation processes; ozone (OZ), peroxone (PO), electrolysis (E), electrolysis/H2O2 (ECP), electroperoxone (EPO), and electrolysis/peroxene/H2O2 (EPOP). Main parameters including contact time, pH, CV concentration, and effect of scavengers were studied. The results showed that all processes were capable of CV decolorization. Among these processes, peroxone and electrolysis/peroxene/H2O2 with efficiency about > 90% were more effective than other ones. A significant decrease in oxidation rate of CV was observed by adding scavengers. This fact was due to higher affinity to react with free radicals. To confirm degradation of CV, FTIR spectra and nitrate ion level were taken. Also, to recognize toxicity the treated wastewater was tested against coliform bacteria. Growths of Escherichia coli in EMB medium were observed. As a result, it confirms that the treated effluent can be discharged to environment.
Similar content being viewed by others
Introduction
The triphenylmethane dyes have been extensively used for histological stain and especially Gram’s Method of classifying bacteria. Also, they are used as a versatile dye in much industrial application like textile processing industries (Parshetti et al. 2011). Crystal Violet (CV), also called as gentian violet, is a species of triphenylmethane colors that is widely used for paper printing, gram staining, detergents, antiseptic (e.g., ringworm and athlete’s foot), anti-freezers, fertilizers, and leather (Harifi-Mood and Hadavand-Mirzaie 2015).
Colored effluents discharging into the environment such as water resources create a major concerning its toxic effect to aquatic flora and fauna (Liu et al. 2004). Therefore, to prevent the adverse effect on biota, they should be treated before disposing to ecosystems. Various techniques including physiochemical and biological processes such as oxidation, advanced oxidation, adsorption, coagulation, membrane filtration, electrochemical processes, and ultrasonic irradiation have been suggested for degradation of synthetic dyes (Chou et al. 1999). Generally, biological processes are less effective on dyes because of inherent characteristics of dyes such as toxicity, high weight, and strong molecular bonds (azo bonds) (Lee et al. 2006). Recently, advanced oxidation processes (AOPs) have been considered as an effective and strong process for wastewater treatment because of the involved oxidative radicals (Comninellis et al. 2008). Ozone and its combination with other oxidation have been recommended for dye and organic material degradation. Under higher pH, ozone is decomposed into hydroxyl radicals leading to the destruction of dyes molecules. However, for enough degradation, the ozonation (OZ) needs more time. To give better results, the ozone is integrated with another oxidation agent (De Witte et al. 2009). One combination method applied for this purpose is a peroxone (PO) process, which uses ozone and peroxide hydrogen. This combination has a strong synergistic effect on pollutants and organic matters that mainly occur due to the generation of hydroxyl radicals (Bakheet et al. 2013). To emphases on the advantage of combination, (Al-Qodah et al. 2018) revealed that the combination of electrochemical with another process enhances the removal efficiency of water pollutants. They provided a good literatures review on increasing better pollutant removal efficiency when ozone-assisted electrocoagulation is used. This process can be integrated with electrolysis by which consumption of hydrogen peroxide can be decreased or even omitted. Referring to the literature, electroperoxone (EPO) significantly has shown the ability to degrade and mineralize organic matter and high weight molecules such as synesthetic dyes. The integration of EC with a free radical producing process has been noted as a new promising and strong procedure (Al-Qodah and Al-Shannag 2019).
Generally, in electrochemical-based process, electrode material, size, and their arrangement are the important parameters affecting the progress of reaction (Adhoum and Monser 2004). Same authors reported that the effectiveness of EC for organic degradation depends on the nature of the anodes and there is a poor current efficiency for traditional electrodes, such as graphite and nickel (Rodgers and Bunce 2001, Feng and Li 2003, Chen 2004). Li et al. demonstrated that the same electrode materials such as Ti/SnO2-Sb, Ti/RuO2, and Pt could be used for the degradation of organic compounds such as phenol (Li et al. 2005). Also, the cathode material is considered by many parameters including lower costs, stability in acidic and alkaline mediums, and low interior resistance for an electrical crossing charge. Chou and et al. have shown that the highest initial current efficiency is provided using steel cathode in contrast with graphite, titanium, and lead. Moreover, using this cathode, greater ferrous ions are produced and subsequently higher degradation rate of organic compounds occurs (Chou et al. 1999).
Platinum and platinum group metals have wide applications because of their unique properties such as stability, high resistance, melting points, and electrical conductivity. Moreover, this group is considered as rare metals and priceless material on the world. Hence, they can be permanently used after application.
The aim of this study is a comparative study to investigate the decolorization of CV based on different AOPs methods including ozone (OZ), peroxone (PO), electrolysis (E), electrolysis/H2O (EP), electroperoxone (EPO), and electrolysis/peroxone/H2O2 (EPOP).
Materials and methods
Standards and reagents
Crystal violet (CAS number: 548-62-9) with empirical formula C25H30ClN3 was prepared from Sigma-Aldrich company (Germany). Other reagents such as methanol (≥ 99.9%), sodium acetate, EDTA, sodium bicarbonate, NaCl, NaOH, HCl, H2O2 (35%) were provided in analytical grade and Merck Group Company (Germany). The chemicals were utilized without further purification.
Experimental setup
The oxidation experiments were carried out in a batch covered cylindrical reactor (Pyrex) with a height of 15 cm and an inner diameter of 10 cm. Ozone was prepared in dry air (Aqua) by an in situ ozone generator with continuously bubbling and a constant dose (2 mg/L.min).
To initiate the electrolysis-based oxidation, a DC power supply (TEK-8051, 30 V, and 5 A double) was used and a constant current of 100 mA was applied. A pair of electrodes, a platinum rod (height of 18 cm and diameter of 2 mm) was used as the anode and stainless steel wool was installed as the cathode with a distance of 2.5 cm from the reactor. The steel wool was fixed on a cylindrical plastic mesh, and platinum was placed. To better mix the reactor contents, a magnet starrier (Alfa, HS-860) was used.
In order to remove the exhausted ozone, an activated carbon Plexi column (with a height of 40 cm and an inner diameter of 6 cm) was designed. To improve the electrical conductivity of the colored solution, 100 mg/L of sodium chloride was added directly as supporting electrolytes.
All experiments were carried out at room temperature (i.e., 22 ± 3 °C). The pH of colored solution was determined using a pH meter (i.e., HATCH), and the desirable pH was adjusted by a 1 M NaOH and 1 M HCl solutions. Finally, to determine the scavenging influences, 100 mg w/v of methanol, sodium acetate, EDTA, and sodium bicarbonate were used under optimal conditions.
Analysis
During decolorization by the OZ, PO, EPO and EPOP processes, samples were collected from the reactor at different reaction times of 5, 10, 15, 20, 25, 30, 45, and 60 min. Then, to avoid the analysis errors from sampling until the determination of CV residuals, 1 mL methanol was interred. This was done due to scavenging the existing free radical in sampled volume and further oxidation. The CV residual concentrations in the solution were measured spectroscopy through UV–visible spectrophotometer (Rayleigh UV 9200, China) at 600 nm. The removal efficiency percentage rate was calculated using the following equation. Also, the apparent decolorization rate was determined based on general first-order kinetics.
To understand the degradation of CV, FTIR spectra were taken using infrared spectrophotometer (Tensor 27, Bruker, Germany). To prepare the samples, a volume of about 500 ml was dried for each run and a dried powder was analyzed.
Toxicity test
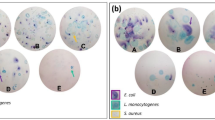
To ensure the reliability of the results, detoxification test of treated wastewater was considered. The experiment was performed by adding the Coliform group bacteria to 0.5 ml of treated wastewater. Then, the bacteria were cultured on EMB agar medium and incubated for 24 h at 35 °C. The Coliform bacteria were harvested from a previously prepared positive EC brace medium. Next, certain amounts of positive sample were transferred to EMB medium. Finally, the growth ability considered as a reduction of CV toxicity. Also, the toxicity of untreated was investigated as blank.
Result and discussion
Time determination
Figure 1 presents the effect of OZ and PO on CV removal efficiency. As can be seen, the PO is so faster and effective than OZ alone. Decolorization of CV using OZ was limited in the first 20 min, and then, the CV concentration was declined exponentially. Additionally, about 40% of the CV was decolorized after 30 min of ozone treatment, while about 80% of the CV was reduced at this time using the PO. In comparison, Wijannarong et al. (2013) reported that only OZ is not able to completely remove reactive dyes such that just about 90% of primary concentration was decolorized at 6 h (Wijannarong et al. 2013). These results possibly occurred due to the delay in ozone decomposition in aqueous solution and consequently postponing the chain mechanism, which is combined with initiation, propagation, and termination (Wijannarong et al. 2013). Simple ozone decomposition and model decomposition were described by (Hoigné et al. 1985, Wijannarong et al. 2013).
Ozone is stable at lower pH (Acar and Ozbelge 2006) and thus inhibits ozone decomposition with regard to unadjusted initial pH (6.8 ± 0.1) of CV solution. Also, the presence of some ions like OH− can enhance the decomposition of ozone by launching the chain mechanism. Therefore, the acidic medium can make a lag phase during OZ process. Furthermore, in the presence of OH−, the ozone decomposition is started as Eq. (2), which takes place very slowly by a constant rate of 70 M−1s−1(Wijannarong et al. 2013).
As previously mentioned, the ozone decomposition is enhanced in the presence of other oxidant agents such as UV, catalyst, or hydrogen peroxide. In the case of hydrogen peroxide, which is used with ozone, the chain reactions initialize. Launching by HO2−, which is arising from partial ionization of hydrogen peroxide, can be described as the following equations (Acar and Ozbelge 2006, Al-Qodah and Al-Shannag 2019):
Because of the higher reaction rate of HO2− (k = 2.8 × 106 M−1s−1) than OH−, PO is more efficient than ozone. The first reaction rate of PO is 40,000 times faster than OZ alone.
To determine the overall decolorization rate of OZ and PO, the first-order kinetic was considered (Rezaee et al. 2016):
where C0 and Ct are the CV concentrations at the beginning of the decolorization process and after time t, respectively. Also, k1 is the constant of first-order kinetics. Figure 1b presents the values of k1 (min−1) and its correlation coefficients (R2). To better understand the reaction rate, the k1 was determined separately for the delay phase of OZ oxidation (0–20 min) and active period time 20–60 min. It is obvious that the delay phase did not have any progress (according to zero slopes). But, later, the rate of OZ oxidation reached 0.0368 min−1. In comparison, the PO was faster and stronger than OZ by a higher amount of k1, i.e., 0.0696 min−1. Also, higher kinetic correlation coefficients (i.e., \( R_{\text{OZ}}^{2} = 0.933 \) and \( R_{\text{OP}}^{2} = 0.996 \) ) indicated good conformity between first-order kinetics and oxidation data. Additionally, according to the k1,OP and k1,oz (for time 0–60 min), it is clear that the peroxene oxidation rate is 2.45 time greater than OZ alone.
At the time of 60 min, the decolorization efficiency was about less than 80% and more than 98% for OZ and PO processes, respectively. Since the greatest removal rate of CV was achieved about 30 min, the end of other experiments was set at 30 min.
Comparison different combination: EC, EP, EPO, EPOP
The oxidation ability of electrolysis and its combination with ozone and hydrogen peroxide were investigated. As shown in Fig. 2, there is the potential oxidation of the electrochemical process and its different arrangements with ozone and H2O2. It is obvious that the combination arrangements provided better results. The combinations of electrolysis with ozone and H2O2 including EP, EPO (electrolysis/O3 or electroperoxone), and EPOP were more effective. The residual amounts of CV at the end of the process for electrochemical, EP, EPO, and EPOP were determined about 45.5 mg/L, 14.6 mg/L, 19.33 mg/L, and 7.36 mg/L, respectively. This result showed that higher decolorization efficiency is provided when using EPO (70%) and EPOP (85.3%).
At an equilibrium relationship, H2O2 dissociation to hydroperoxide ions (HO2−) can be described as Eq. (12) (Acar and Ozbelge 2006). However, in the presence of a sufficient amount of H2O2, the equilibrium produces more HO2−. Since the needed hydrogen peroxide for EPO process is generated from electrolysis, its generation rate is limited. As a result, the better degradation of CV was observed when adding hydrogen peroxide externally.
Moreover, to achieve an efficient decolorization, the appropriate ratio of H2O2 to ozone should be available. However, productive or external H2O2 can be as main limiting agent for PO and peroxone-based reactions. Also, it is of note that the excessive amount of hydrogen peroxide acts as a scavenger and consequently the efficacy of process due to self-consumption of free oxidative radical is diminished (Glaze 1987). Although among the electroperoxone processes the highest efficiency was related to EPOP, the classic PO showed better performance. This fact can result from the adequate and adjustable ratio of H2O2 to O3 and also the absence of electrolysis products such as oxygen or undesirable released anodic materials.
The effect of different parameters
Effect of pH
The pH is one of the main controlling parameters of the chemical and biochemical treatment processes such as advanced oxidation and biological processes. Figure 3a and b shows the effect of initial pH and its role in PO and EPOP processes.
As shown in Fig. 3a, it is evident a higher pH (pH 7 and 9) is more effective on the oxidation processes and more than 98% of removal efficiency was obtained at time of 5 min. Also, it can be seen that at pH 5 the PO has the lowest efficiency with about 50% removal efficiency.
According to Fig. 3b, the maximum dye degradation was 82.7% when the pH was adjusted at 9. Because the CV has two acid dissociation constants (i.e., pKa1 5.31 and pKa2 8.64), it is expected that the decolorization of CV at higher than these amounts is more effective. Therefore, higher removal of CV at basic pH matches with dissociation constants. Also, the cationic structure of the CV favors decolorization at higher pH values (Sihem et al. 2008). Acar and Ozbelge (2006) reported determined the maximum and lowest ozone concentrations at pH 2.5 and pH 10, respectively (Acar and Ozbelge 2006). Also, they found that a faster decomposition rate of ozone is related to basic pH in an aqueous medium. These results demonstrate that the basic pH can prompt the PO-based oxidation processes. Zhang et al. (2010) showed that with an increase in the pH value, the rate of H2O2 decomposition increased and, as a result, more free radicals would be generated. This fact can be described through Eqs. 13 and 14:
Also, it can be noted that at low pH levels, protons can be scavenged by the hydroxyl radical as Eq. (15), leading to a decrease in CV decolorization rate.
Effect of initial CV concentration
The effect of initial CV concentration in PO and EPOP processes is shown in Fig. 4. The results demonstrate that the PO is more effective on CV removal rather than the EPOP process. Figure 4a illustrates the ability of the PO process on a different level of CV concentrations. According to this figure, at the end of the considered time of 30 min, all concentrations (25–200 mg/L) were decolorized approximately. However, the EPOP process was not able to degrade CV under the same condition. In comparison, the removal efficiency percentage was determined for EPOP process about 61 to 90%, where the overall decolorization efficiency for PO was more than 98%.
Based on Fig. 4b, it is seen that the removal rate of CV is not limited by dye concentrations, owing to the same oxidation pattern of all concentrations (25 to 200 mg/L). Also, the removal pattern for each process is a different one from another. These results revealed that the amounts of available H2O2 and consequently OH2− are limiting factors in this process. As a result, it observed that the PO is able to decolorize any interring concentration and there limiting factor can be restricted to mixing power and mass transfer of CV.
As shown in Fig. 4c, with an increase in CV concentration, an exponential and a linear decrease in the decolorization rate and the apparent rate constant (kapp, min−1) for PO and EPOP were observed, respectively. The kapp of the CV decolorization of EPOP at dye concentrations 25, 50, 100, and 200 mg/L was 7.32 × 10−2, 6.10 × 10−2, 2.69 × 10−2, and 2.87 × 102 min−1, respectively. Also, the kapp for PO process was determined about 6.06 × 10−1, 1.68 × 10−1, 1.27 × 10−1, and 1.09 × 10−1 min−1. It is obvious that the classical PO is more effective on CV oxidation.
Effect of scavengers
To investigate the effects of free radical scavenging agents, common organic/inorganic scavengers such as methanol, EDTA, acetate, and sodium bicarbonate were considered. Figure 5 presents the behavior of PO and EPOP processes in the presence of scavengers in comparison with blank. The results indicate that the added organic matter consumes the free radicals and thus lowers the decolorization rates. This negative effect was significantly affected by EPOP processes. The removal efficiency of EPOP process was lost about 38% to 54%, in the presence of scavengers. Among the tested materials, methanol and EDTA showed more affinity to consume free radicals. In the PO process, despite observing the negative effect of organic matter, they had a relatively short effect on primary time the PO operation. The scavenging effects of organic matter were in the same way for both processes. Moreover, sodium bicarbonate created the lowest scavenging manner compared to the other agents. The decline in oxidation in the presence of scavengers is related to their higher affinity to react with free radicals. The reaction rate of generation of hydroxyl radical (Eq. 6) is about 1.1 × 105 M−1 s−1. In comparison with the reaction rate of hydroxyl radical with organic matter (n × 107 - n × 109 M−1 s−1), it can be concluded that a part of generated radicals is consumed by scavengers. Table 1 shows the reaction constant rate of hydroxyl radical with considered scavengers in the present study. Accordingly, the all considered matters could be nominated as scavengers in the presence of radical-based processes.
Degradation conformity test
FTIR
In order to understand about CV degradation via different oxidation processes, FTIR analysis was considered. Figure 6 illustrates the results of this test. Comparing the FTIR spectrum of degraded dye with control or untreated solution clearly reveals that the CV was significantly undergrounded by ozone and other oxidative processes. Statistically significant differences between the FTIR spectra of treated solutions and control imply the occurrence of degradation. According to the FTIR plots, noticeable variations are seen in both fingerprint and functional group regions of 3500 to 500 cm−1 of the oxidized samples in comparison with control. These noticeable variations occurred for all oxidation processes (Ameen et al. 2013). The sharpest peaks for the untreated sample were mono-substituted and para-disubstituted benzene rings, which support the peak at 1583 cm−1. This peak is indexed to the C = C stretching of the benzene ring. Also, peaks at 1164 cm−1 and 2920 are indexed to the C–N stretching vibrations and C–H stretching of the asymmetric CH3 group, respectively. The peak for the C–N stretch of aromatic tertiary amine appeared at 1360 cm−1. Moreover, the absence of N = N stretching vibrations peak at 1360 cm−1 can be linked to breaking the azo bond. After oxidation of CV, the region between 937 and 516 cm−1 disappeared, probably because of the decomposition of aromatic rings.
The level of nitrate
To better understand the oxidative degree, the inorganic intermediates like nitrogen compounds (e.g., nitrate and ammonia) can be determined. According to Fan et al. (2009), when the CV is degraded by Fenton and Fenton-like processes, many organic and inorganic intermediates can appear (Fan et al. 2009). These researchers reported degradation intermediates and mineralization pathways of CV. When the CV undergoes an oxidative process, some organic intermediates such as aniline, acetic acid, 4-aminophenol, pararosaniline, aminobenzene, and other aromatic compounds may possibly emerge. Furthermore, at the end of the oxidation process, CO2, N2, SO42−, NO3−, and H2O were produced through the mineralization process. The presence of this species implies high degradation of target organic compound. Therefore, at this step, the nitrate content was determined at the end of the experiment. The results of this test (Fig. 7) show the occurrence of mineralization. The nitrate concentrations were determined from 0.5 to up then 1 mg/L. In the case of PO, the fewer amount of nitrate possibly is not related to its low degradation degree. This result can be attributed to different degradation pathways. In addition, it has been reported that the ozonation of some compounds with aromatic structures increases their toxicity (Shang and Yu 2002).
Toxicity test
Triphenylmethane dyes like CV are remarkably used for a wide range of industrial and medical applications. Also, because of their good antimicrobial characteristics and photocytotoxicity properties, they are considered intensively by photodynamic therapy researchers (Fan et al. 2009). In addition, it has been reported that the ozonation of some compounds with aromatic structures increases in toxicity (Shang and Yu 2002). Therefore, CV can be impacted by chemical oxidation and its antimicrobial characteristics are degraded. For better understanding, this variation, the toxicity test was carried out. As presented in Fig. 8, antimicrobial power of CV changes by the formation of Escherichia coli colonies on the EMB medium. Accordingly, it is clear that significant colony growth exists for all treated samples compared to the untreated sample. However, the fine colony of E. coli appeared in the control plate that can occur due to the relatively low effect of CV toxicity on E. coli. Nevertheless, the important key is the appearance of colonies in the samples exposed to degraded ones. The by-products of a degraded CV such as aniline, benzylamine, and 4-aminobenzoic acid are more toxic than a CV, suggesting a decrease in the overall toxic storage. This result can assure the possible discharging of treated effluent.
Conclusion
In this study, the effect of ozonation, electrolysis, electrolysis/H2O2, peroxone, electro-peroxene, and electro-peroxene/H2O2 was examined for decolorization of CV from aqueous solutions. Results showed that peroxone and electroerosion/H2O2 were more effective than other studied compounds. To investigate the effects of radical scavengers, the methanol and EDTA had more affinity to scavenge the free hydroxyl radical. The qualitative and quantitative analyses of intermediates compounds by FTIR and nitrate content showed the occurrence of CV degradation. Finally, according to toxicity test and growth of Escherichia coli colonies, it can be concluded that the CV by-product can be discharged to the environment.
References
Acar E, Ozbelge T (2006) Oxidation of Acid Red-151 aqueous solutions by the peroxone process and its kinetic evaluation. Ozone Sci Eng 28(3):155–164
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process 43(10):1281–1287
Al-Qodah Z, Al-Shannag M (2019) On the Performance of Free Radicals Combined Electrocoagulation Treatment Processes. Sep Purif Rev 48(2):143–158
Al-Qodah Z, Al-Shannag M, Bani-Melhem K, Assirey E, Yahya MA, Al-Shawabkeh A (2018) Free radical-assisted electrocoagulation processes for wastewater treatment. Environ Chem Lett 16(3):695–714
Ameen S, Akhtar MS, Nazim M, Shin H-S (2013) Rapid photocatalytic degradation of crystal violet dye over ZnO flower nanomaterials. Mater Lett 96:228–232
Armstrong W (1969) Relative rate constants for reactions of hydroxyl radicals from the reaction of Fe(II) or Ti(III) with H2O2. Can J Chem 47(20):3737–3744
Bakheet B, Yuan S, Li Z, Wang H, Zuo J, Komarneni S, Wang Y (2013) Electro-peroxone treatment of Orange II dye wastewater. Water Res 47(16):6234–6243
Buxton GV, Elliot AJ (1986) Rate constant for reaction of hydroxyl radicals with bicarbonate ions. Int J Radiat Appl Instrum. Part C. Radiat Phys Chem 27(3):241–243
Campbell I, McLaughlin D, Handy B (1976) Rate constants for reactions of hydroxyl radicals with alcohol vapours at 292 K. Chem Phys Lett 38(2):362–364
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41
Chou S, Huang Y-H, Lee S-N, Huang G-H, Huang C (1999) Treatment of high strength hexamine-containing wastewater by electro-Fenton method. Water Res 33(3):751–759
Comninellis C, Kapalka A, Malato S, Parsons SA, Poulios I, Mantzavinos D (2008) Advanced oxidation processes for water treatment: advances and trends for R&D. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 83(6):769–776
De Witte B, Dewulf J, Demeestere K, Van Langenhove H (2009) Ozonation and advanced oxidation by the peroxone process of ciprofloxacin in water. J Hazard Mater 161(2–3):701–708
Fan H-J, Huang S-T, Chung W-H, Jan J-L, Lin W-Y, Chen C-C (2009) Degradation pathways of crystal violet by Fenton and Fenton-like systems: condition optimization and intermediate separation and identification. J Hazard Mater 171(1–3):1032–1044
Feng Y, Li XY (2003) Electro-catalytic oxidation of phenol on several metal-oxide electrodes in aqueous solution. Water Res 37(10):2399–2407
Glaze WH, Kang JW, Chapin DH (1987) The chemistry of water treatment processes involving ozone hydrogen peroxide and ultraviolet radiation. Ozone Sci Eng 9(4):335–352
Harifi-Mood AR, Hadavand-Mirzaie F (2015) Adsorption of basic violet 16 from aqueous solutions by waste sugar beet pulp: kinetic, thermodynamic, and equilibrium isotherm studies. Chem Speciat Bioavailab 27(1):8–14
Höbel B, von Sonntag C (1998) OH-radical induced degradation of ethylenediaminetetraacetic acid (EDTA) in aqueous solution: a pulse radiolysis study. J Chem Soc Perkin Trans 2(3):509–514
Hoigné J, Bader H, Haag W, Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water—III. Inorganic compounds and radicals. Water Res 19(8):993–1004
Lee J-W, Choi S-P, Thiruvenkatachari R, Shim W-G, Moon H (2006) Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm 69(3):196–203
Li X-Y, Cui Y-H, Feng Y-J, Xie Z-M, Gu J-D (2005) Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes. Water Res 39(10):1972–1981
Liu W, Chao Y, Yang X, Bao H, Qian S (2004) Biodecolorization of azo, anthraquinonic and triphenylmethane dyes by white-rot fungi and a laccase-secreting engineered strain. J Ind Microbiol Biotechnol 31(3):127–132
Parshetti G, Parshetti S, Telke A, Kalyani D, Doong R, Govindwar S (2011) Biodegradation of crystal violet by Agrobacterium radiobacter. J Environ Sci (China) 23(8):1384–1393
Rezaee A, Pourtagi G, Hossini H, Loloi M (2016) Microbial cellulose as a support for photocatalytic oxidation of toluene using TiO 2 nanoparticles. J Appl Polym Sci 133(8):43051
Rodgers JD, Bunce NJ (2001) Electrochemical treatment of 2, 4, 6-trinitrotoluene and related compounds. Environ Sci Technol 35(2):406–410
Shang N-C, Yu Y-H (2002) Toxicity and color formation during ozonation of mono-substituted aromatic compounds. Environ Technol 23(1):43–52
Sihem A, Kamel D, Halima C, Tahar S, Abdlkader B, Azzedine RD (2008) Elimination of a cationic dye (Crystal violet) in aqueous medium by TiO2/UV oxidation process. Asian J Chem 20(7):5581
Wijannarong S, Aroonsrimorakot S, Thavipoke P, Sangjan S (2013) Removal of reactive dyes from textile dyeing industrial effluent by ozonation process. APCBEE procedia 5:279–282
Zhang H, Wu J, Wang Z, Zhang D (2010) Electrochemical oxidation of crystal violet in the presence of hydrogen peroxide. J Chem Technol Biotechnol 85(11):1436–1444
Acknowledgement
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences (Grant Number: 97061) for the financial support. The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdi, M., Balagabri, M., Karimi, H. et al. Degradation of crystal violet (CV) from aqueous solutions using ozone, peroxone, electroperoxone, and electrolysis processes: a comparison study. Appl Water Sci 10, 168 (2020). https://doi.org/10.1007/s13201-020-01252-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01252-w