Abstract
Results of the interaction of process variables and the consequential mixture of phenolic compounds adsorption study are expected to shed brighter light on the wastewater treatment applications. Accordingly, the aims of this research are to model and optimize the process variables which impinged on the simultaneous adsorption of phenol and 4-chlorophenol (4-CP) in the binary solution by spherical activated carbon (SAC). Batch assessments were designed using response surface methodology software. The process variables, namely SAC dosage and pH were varied over the 1.50–3.50 g/L and 4.00–9.00 g/L ranges, respectively, were experimented. The analysis of variance results showed the significant models could precisely predict the percentage removals of phenol and 4-CP, indicating models reliability. The interaction of process variables was inconspicuous for the case of phenol adsorption. However, increasing the pH would deteriorate the 4-CP adsorption which was partially offset by raising the SAC dosage. Considering the environmental benefits, optimization taken place at the SAC dosage and pH of 3.50 g/L and 7.60 g/L, respectively, was selected. By employing the optimized conditions of SAC dosage of 3.50 g/L at pH 7.60 for the adsorption process, the predicted phenol and 4-CP removal percentages were found to be 85.4 % (73.1 mg/g) and 96.2 % (82.6 mg/g), respectively, which were in agreement with the experimental runs.
Similar content being viewed by others
Introduction
Contamination of fresh water by phenol and its derivatives due to their extensive applications has become a serious global predicament. The introduction of phenolic compounds to the receiving water bodies is significantly harmful to the environment because of their toxicity, recalcitrant and bioaccumulation properties (Dabrowski et al. 2005). To highlight further, these compounds are also suspected to be carcinogenic and mutagenesis to living organisms and hence, World Health Organization (WHO 1994) has set a limit of 1 mg/L in regulating the phenol concentration in drinking water.
Recently, the release of wastewaters containing phenolic compounds to the environment becomes inevitable due to the widespread use in many industrial processes (Dabrowski et al. 2005). In 1989, the annual industrial production of chlorophenols was estimated at 200,000 tons (WHO 1989; Tobajas et al. 2012). Out of this, approximately 190 ton/month of phenolic wastes were disposed primarily by petrochemicals, pharmaceuticals and polymer industries (Zareen and Anjaneyulu 2005). In the United States alone, the quantity of phenol and chlorophenols wastes produced in industries were amounted to 56,000 and 1900 tons, respectively, in 1999 (Tarighian et al. 2003; Tobajas et al. 2012). The industrial wastewaters are characterized by variable concentrations of phenolic compounds, generally ranging between 500 and 4000 mg/L (Tobajas et al. 2012). In a coal processing industry, the produced wastewater can contain phenol concentration up to 6800 mg/L (Busca et al. 2008; Dotto et al. 2013). In the case of mono-chlorophenols, the effluents concentration range from non-detectable to 20 mg/L has been reported by WHO (1989). In light of the phenolic compounds content in industrial wastewaters, treatment of these streams is essential in order to alleviate the toxicity effects that are detrimental to the environment upon the disposal.
In line with this necessity, biological treatment system via aerobic and anaerobic processes has been frequently employed to treat wastewater containing phenolic compounds due to its economical attraction (Valo et al. 1990; Lim et al. 2013a). However, as phenolic compounds are usually toxic and recalcitrant in nature, the microorganisms present in activated sludge process are often inhibited and incompetent to bioremediate wastewater containing high concentration of phenolic compounds (Sahinkaya and Dilek 2007; Lim et al. 2012). To top it off, typically, lengthy steady state of acclimation period is required before the activated sludge could be used to treat wastewater laden with phenolic compounds (Lim et al. 2013b). Owing to these limitations, physical and chemical techniques used to treat wastewater containing phenolic compounds have gained increasingly recognition among the researchers (Xiong et al. 2011). Among these techniques, the popularity of adsorption process continues unabated for years to separate phenolic compounds from the wastewaters (Dabrowski et al. 2005; Sunil and Jayant 2013). Besides its simplicity in design, the adsorption of adsorbate onto the activated carbon has been found to outperform the other physicochemical methods in terms of its capability of successfully adsorbing a broad range of adsorbates (Foo and Hameed 2009; Shehzad et al. 2015, 2016). In view of this matter, the competency of activated carbon could be justified by its characteristics such as large surface area, micro-porous structure, high adsorption capacity and large surface chemical nature which result in extensive degree of surface reactivity (Quintelas et al. 2006; Bharathi and Ramesh 2013). The use of rubber tire activated carbon to treat phenolic-bearing wastewater was delineated by Gupta et al. (2014) which owned adsorption capacities of 71 mg/g for p-cresol and 48 mg/g for phenol. In the study implemented by Yang et al. (2014) modification of activated carbon via amination process had brought about the enhancement of phenol adsorption capacity which was attributed to the strengthened π–π dispersion between phenol and basal plane of activated carbon by pyridine, pyrrolic N. Furthermore, since years ago, the exploitation of powdered and granular activated carbons to adsorb various phenolic compounds had been documented by many researchers (Hamdaoui et al. 2003; Ivancev-Tumbas et al. 2008; Shao et al. 2013).
The industrial wastewaters generally contain phenol and its derivatives simultaneously. Hence, from a practical point of view, the results of adsorption from binary or multisolute systems are more relevant. Among those reported activated carbon adsorption works on multisolute systems were simultaneous removal of phenol, p-chlorophenol and p-nitrophenol by Kim et al. (1985), phenol and m-cresol by Leitao and Serrao (2005), phenol and resorcinol by Kumar et al. (2011), phenol and m-chlorophenol by Toth et al. (2012) and p-cresol and p-nitrophenol adsorption equilibria by Nouri (2002). Despite there are numerous reported works on the adsorption of multisolute phenolic components, only little information is however, available in literature with regard to the simultaneous removal of phenol and 4-chlorophenol (4-CP) from their binary mixture via adsorption by activated carbon. Therefore, research attempts aiming toward this direction are deemed crucial to offer more understanding for plausible future applications in the wastewater treatment plants.
In general, the adsorption of solutes from aqueous solution by any adsorbent is influenced by many factors, e.g., initial concentration of adsorbate, adsorbent dosage, contact time, pH, intensity of agitation, etc. The sequential optimization of single factor at a time as carried out traditionally often overlooks interactive effects of various factors on the adsorption process, in addition to the requirement of large number of experimental runs which are costly and time-consuming. These drawbacks, however, can be resolved by the statistical design of experiments, which reduce the number of experiments and provide an appropriate model for process optimization. In this regard, response surface methodology (RSM) plays an important role to analyze and determine the optimum condition of interactive factors within the design space of the experimental study (Myers et al. 2009).
The main objective of this work is to optimize the interactive factors involve in the simultaneous adsorption of phenol and 4-CP by spherical activated carbon (SAC) using central composite design (CCD) of RSM. SAC was selected in this study due to considerable various advantages over the other forms of activated carbon such as extremely low resistance to liquid diffusion, higher adsorption efficiency, more resistance to abrasion and better mechanical properties. Subsequently, models concerning adsorption of phenol, 4-CP and mixture of these phenolic compounds in terms of COD are as well developed to anticipate their removals amidst the presence of variable factors.
Materials and methods
Materials
The SAC with a trade name of ‘SPHERICAL SHIRASAGI X7100H (DRY)’ was purchased from Japan EnviroChemicals, Ltd. Originally, SAC is a coal based spherical activated carbon and being produced through steam at high temperature. Being designed for wastewater treatment, the standard specifications of SAC as obtained from manufacturer are tabulated in Table 1. The physico-chemical characteristics of SAC as determined in laboratory are also shown in Table 2. The adsorption capacities of phenol and 4-CP by SAC were separately measured at natural pH (Table 2).
The solids phenol and 4-CP used were purchased from Merck Chemicals (Malaysia) and of synthesis grade with >99 % purity. Stock solutions containing 1000 mg/L of phenol and 4-CP were prepared by dissolving these solids in distilled water and separately stored in an amber glass bottle prior to use by dilution to the required concentration.
Adsorption study procedures
The simultaneous adsorption of phenol and 4-CP to SAC in their binary solution was investigated via batch experiments. All trials were completed by shaking 20 mL of binary solution containing 300 mg/L of phenol and 4-CP each in well capped 25-mL cylindrical flasks with a diameter of approximately 30 mm for a period of 24 h (equilibrium time length attainment). The orbital shaker, model IKA KS Basic 260, was utilized to provide a shaking speed of 250 rpm and the experiments were performed at the temperature of 25 ± 1 °C. The pH for all experimental runs were adjusted using 1 M of HCl and 1 M of NaOH with SAC dosage being weighted accurately to 0.005 g. The interactive factors or process variables namely, SAC dosage and pH, which affected the simultaneous adsorption of phenol and 4-CP to SAC were evaluated with the help of RSM software. Immediately after each run, the binary solution was examined for phenol, 4-CP and COD concentrations. The COD value was mainly contributed by both unadsorbed adsorbates (phenol and 4-CP concentrations in binary solution). The removal efficiencies of phenol, 4-CP and COD from the binary solution were subsequently calculated via the following equation:
where C i and C f are the initial and final concentrations of phenol, 4-CP or COD. Each adsorption study was triplicated with average removal efficiency percentages for phenol, 4-CP and COD being reported.
Chemical analysis
The concentrations of phenol and 4-CP in the binary solution were analyzed using a UV–Vis spectrophotometer, model Shimadzu U-2600. The method of quantification followed closely the procedure outlined by Khan et al. (1997). In enhancing the accuracy and precision of measurement, the structures of phenol and 4-CP were first converted into the molecular form via acidification with 1 M of HCl solution for both blank and sample. The concentrations of phenol and 4-CP in the binary solution were then obtained as the function of absorbance with regard to the λ max of phenol and 4-CP at 270 and 280 nm, respectively. Their concentrations were subsequently calculated from the Eqs. (2) and (3) which were substituted with the measured absorbance values of the sample and solved simultaneously:
where A 270 and A 280 are the absorbance at λ max of phenol and 4-CP, respectively, C 1 and C 2 are the concentrations of phenol and 4-CP, respectively, and b is the non-variable path length of sample fixed at 1 cm. Next, the ε 11 and ε 12 represent the molar extinction coefficients of phenol and 4-CP at λ max of 270 nm, respectively, while ε 21and ε 22 represent the molar extinction coefficients of phenol and 4-CP at λ max of 280 nm, respectively. The molar extinction coefficients of phenol and 4-CP at each λ max were preliminary acquired from the standard calibration runs. Their values were 0.0151 and 0.0095 L/mg cm with respect to ε 11 and ε 12 and 0.0063 and 0.0137 L/mg cm with respect to ε 21and ε 22.
The concentration of COD was measured in accordance with the Standard Methods for the Examination of Water and Wastewater (APHA et al. 2012). In general, the standard K2Cr2O7 digestion solution containing sample was refluxed in a close vessel for 2 h at 150 °C before being cooled to room temperature. The COD concentration in the sample was then determined by titrating with the standard ferrous ammonium sulfate (FAS) titrant.
Experimental design
The Design-Expert® Version 8.0.7.1 (Stat-Ease, Inc., Minneapolis, MN 55413) software was employed to render a platform for RSM to analyze and optimize the simultaneous adsorption of phenol and 4-CP to SAC. The most widely exploited approach of RSM, i.e., central composite design (CCD), was used to evaluate the correlation between the process variables and the responses (Bashir et al. 2011). Vindicated by Guven et al. (2008) CCD is an effective design tool that is ideal for sequential experimentation as it permits reasonable volume of information to test lack of fit when a sufficient number of experimental values exist. The significant process variables considered in this study were SAC dosage (A) and pH (B) as demonstrated in Table 3. Each process variable was numerically varied over three levels between −1 and +1 at the real value range determined from the preliminary experiments. The monitored responses resulted from the interactive effects of process variables were the percentage removals of phenol, 4-CP and COD from the binary solution. The design of experimental conditions based on CCD is illustrated in Table 4. The total numbers of experiments from the consequential two process variables were attained as 13. Eight experiments were augmented with five replications at the center point of each process variable to fit the second-order polynomial response surface model of quadratic equation and assess the pure error (Bashir et al. 2010, 2011). According to Myers et al. (2009) the CCD design stands on second-order polynomial response surface model is primarily used for process optimization.
Basically, the relationship between the input of process variable and the response is given as:
where Y is the response, f is the unknown function of the response, x 1, x 2, x 3… x k are the input of process variables that can affect the response, k is the number of process variables and \(\varepsilon\) is the random error component that represents other sources of variability not accounted for by f (Montgomery 2008; Bashir et al. 2011). The Taylor series expansion of Eq. (4) through second-order terms, contain all model terms through order two, would result in a model of the type (Myers et al. 2009):
The Eq. (5) could be simplified to:
where Y is the response, x i and x j are the process variables, β 0 is the constant coefficient, β i , β ii and β ij are the interaction coefficients of linear, quadratic and second order terms, respectively, k is the number of process variables and \(\varepsilon\) is the random error component. The matrix notation of the model is given as (Bas and Boyaci 2007):

Because each process variable was varied into three levels (Table 3), CCD commended quadratic model (Eq. (6)) in assessing the interactive effects of process variables and the consequential results of responses, in addition to predict the optimum conditions.
Analysis of variance (ANOVA) was later used for graphical analyses of the data to obtain the interaction between the process variables and the responses. The quality of the fitted quadratic model was expressed by the coefficient of determination (R 2) and its statistical significance was verified by the F value (Fisher variation ratio) and Adequate Precision (Bashir et al. 2010). The instantaneous consideration of multiple responses involved the initial creation of a suitable response surface model and subsequently, identification of a set of operational condition that optimized the targeted response such in the most desired range (Bashir et al. 2010).
Results and discussion
The interaction of process variables, SAC dosage and pH in this case, were evaluated in which CCD of RSM was used as a core software kit in observing the simultaneous adsorption of phenol and 4-CP to SAC. The results obtained from the present study are comprehensively discussed after this section.
Analysis of response surface models of adsorption using ANOVA
The simultaneous adsorption of phenol and 4-CP by SAC advanced collectively with the removal of COD from the binary solution and their respective experimental results are tabulated in Table 5. From the experimental run, it could be seen that the adsorption of phenol varied over a broader range than 4-CP, correspondingly from 34.1 to 89.5 and 85.9 to 100.0 %. As COD was virtually the sole contribution of both phenol and 4-CP in the binary solution, its removal efficiency localized between their ranges, i.e., 57.8–94.8 %.
The CCD renders an empirical design in relating interactive process variables with responses via the quadratic equation. The quadratic models following the second-order polynomial response surface equation for each response can be derived by applying the factorial regression analysis on the experimental data (Table 5). Upon eliminating the insignificant model terms, the final response regression models in terms of coded process variables are given as below:
Also, the derivations in terms of actual process variables for the final response regression models can be represented as follows:
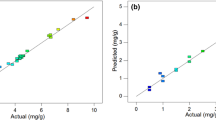
The results of ANOVA for the final response regression models are illustrated in Table 6. Only the statistically significant model terms are incorporated into the final response regression models in order to achieve a good fit. The Prob > F values are all less than 0.0001 which herald the models are significant. The Prob > F value of less than 0.05 indicates the model significance. However, the value greater than 0.10 indicates the model developed is inadequate. The F test of lack of fit describes the variation of the data scattering the fitted model. Presuming the model does not fit the data well, a significant lack of fit will ensue; suggesting that there may be some systematic variation unaccounted by the hypothesized model (Bashir et al. 2010). On the contrary, the larger Prob > F values for the lack of fit (>0.05) demonstrate that the F-statistic is inconsequential, implying significant correlation of model between the process variables and responses as revealed in Table 6 (Ghafari et al. 2009). The R 2 coefficient discloses the quality of fit of a model. Suggested by Joglekar and May (1987) for a good fit of a model, R 2 value should be at least of 0.8. A high R 2 value, close to 1.0, is desirable and a reasonable agreement with the adjusted R 2 is necessary. In addition, Table 6 also shows that the predicted R 2 values are in reasonable conformity with the adjusted R 2 values for all the developed models. There is merely less than 2 % of the total dissimilarity might not be explained by the models for all cases. The adequate precision (AP) assesses the signal-to-noise ratio. The ratio greater than 4.0 denotes sufficient model favoritism. The coefficient of variation (CV) which is computed as the percentage of the standard deviation to the mean value signifies the model reproducibility. The model CV of less than 10 % is typically assumed to be reproducible. This criterion has been fulfilled by all the models of this study (Table 6). Finally, to vindicate if the developed models provide an adequate approximation to the real system, the diagnostic plots of predicted versus actual values can be exploited to judge the model satisfactoriness. Figure 1 shows the reading of actual values scattered closely the line of predicted values for the removals of phenol, 4-CP and COD which underscored the agreement between the real data and the ones gained from the models. A further attempt to substantiate is projected in Table 5 which points up the negligible readings of standard deviation calculated from the observed and predicted values of phenol, 4-CP and COD removals.
Interaction study of process variables
The interaction of process variables and the consequential adsorption of phenol, 4-CP and COD by SAC from the binary solution are presented in Fig. 2. While phenol was being adsorbed, the process variables debunked inconspicuous interaction, shown by the incoherently segregated curves in Fig. 2a. The intentions to expose the effects of individual process variable on phenol removal are highlighted in Fig. 3a. This perturbation plot shows SAC dosage played a major role in removing phenol since a sharp curvature could be noticed as compared with pH presented as almost plateau. In fact, the models with regard to phenol removal [Eqs. (8) and (11)] only include model term of SAC dosage.
Next, the interaction of process variables and the resulting removal of 4-CP by adsorption to SAC is demonstrated in Fig. 2b. The interaction of SAC dosage and pH could be observed at their respective coded levels from 0 coded units (Table 3) onward. Likewise to phenol removal, SAC dosage was once again responsible for the enhancement of 4-CP adsorption in comparison with pH as projected in Fig. 3b which exhibits a sharp curvature belongs to SAC dosage. Nevertheless, at the coded levels of beyond 0 coded units, a slight drop of nearly plateau pH curvature could be detected; representing a decrease of 4-CP adsorption at higher pH. This phenomenon could be justified by the pK a value of 4-CP of 9.37 at 25 °C (Dabrowski et al. 2005). The increase of pH will dissociate the 4-CP molecular structure forming ionic structure of 4-CP (with −1 charge after losing its H+ ion). This ionic structure of 4-CP is naturally unfavorable to be adsorbed by SAC as opposed to its molecular structure form plausibly due to the SAC surface repulsion effect as a result of charge similarity. Conversely, similar occurrence was not exuded by phenol as shown in Fig. 3a because phenol possesses higher pK a value of approximately 10.00 at 25 °C (Dabrowski et al. 2005). Thus, more resistant to the dissociation at the experimented pH range. In the matter of 4-CP adsorption, hence, the increase of SAC dosage would interact with pH at coded levels of above 0 coded units by partially offsetting the adverse effect of pH.
Lastly, as the present of COD was mainly contributed by phenol and 4-CP, the interaction of process variables which led to the removal of COD was virtually all depending on the SAC uptake of phenol and 4-CP in the binary solution. The interaction of process variables as above-mentioned for 4-CP (Fig. 2b) could also be manifested in Fig. 2c in which the partial overlapping of SAC dosage and pH interaction curves occurred at the coded levels greater than 0 coded units. Likewise with the adsorption of phenol and 4-CP, the adsorption of COD was as well proportionally influenced by SAC dosage as compared with pH (Fig. 3c). Furthermore, as the adsorption of phenol remained unaffected by the experimented pH range (Fig. 3a) and 4-CP decreased with increasing pH (Fig. 3b), an intermediate flop of pH curvature also could be detected as shown in Fig. 3c.
Adsorption capacities of SAC at different conditions of interactive process variables
The capabilities of SAC to simultaneously adsorb phenol and 4-CP at different conditions of interactive process variables are presented in Fig. 4, showing the three-dimensional response surface and their respective contour plots. As for the adsorption of phenol, a maximum removal of nearly 90 % could be attained at the SAC dosage of 3.5 g/L and decreased with the reduction of SAC dosage irrespectively to the pH effect of the experimented range. However, a complete removal of 4-CP by SAC was possible and anticipated to transpire at the SAC dosage of between 3.0 and 3.5 g/L and pH below 9.0 as accentuated by the peak in Fig. 4b. Similar observations were noticed by Rawajfih and Nsour (2006) in which up to 88 and 100 % of phenol and 4-CP were adsorbed by surfactant-modified bentonite. As a whole, the adsorption of 4-CP by SAC was always higher than phenol, considered in terms of the highest removal efficiency, for all the conditions of interactive process variables studied. This could be rationalized by the solubility approach in which the solubility of phenol is higher than 4-CP, 93 and 27 g/L, respectively, in the aqueous solution at 25 °C (Dabrowski et al. 2005). According to the Toth et al. (2012) a poor soluble solute would own weak interaction with the solvent. The additional of chloro-group to the phenol structure curtailed its interaction energy with water, thereby permitting higher degree of hydrophobic interaction with surface of adsorbent (Rawajfih and Nsour 2006). Hence, in the binary solution, the 4-CP with lesser degree of solubility relative to phenol would be adsorbed to a greater extent by SAC. Secondly, the presence of chloro- group, an electron withdrawing group, in the 4-CP molecule will also reduce the electron density of aromatic ring which consequentially beefed up the interaction of phenol ring and SAC (Deng et al. 1997). To substantiate further, the adsorption capacity (Q m ) obtained from the Langmuir isotherm model plots for phenol and 4-CP (Fig. 5) were 213 and 251 mg/g, respectively, which were in conformity with the solubility trends whereby 4-CP is less soluble than phenol and tends to be more readily adsorbed by the SAC. For the adsorption of COD, a removal efficiency ranging from 58 to 95 % as shown in Fig. 4c falls within the lowest value of phenol and highest value of 4-CP removal efficiency ranges. Again, this was by virtue of phenol and 4-CP that contributed to COD in the binary solution.
Optimization of interactive process variables conditions
The optimization of interactive process variables conditions were predicted using CCD of RSM software. Initially, each process variable was independently increased or decreased within the designed range in an attempt to search for the maximum responses. Subsequently, the combination of these process variables was selected as the condition to achieve the aim. The software also combined the individual desirabilities of process variables into a single number in the search to maximize the function. Accordingly, there are nine possible optimum conditions were predicted with the maximum removals of phenol, 4-CP and COD ranging from 85.4 to 88.6, 96.2 to 100.0 and 90.9 to 94.7 %, respectively (Table 7). The values of desirability obtained for all the solutions were close to 1.0, demonstrating maximum desirability. For the benefits of the environment, the discharged of treated industrial wastewater is commended to have pH within the range of 6.00–9.00 (Malaysia Environmental Quality Act 2000). For this reason, the solution-9 with the operating condition integrated with 3.5 g/L of SAC dosage and binary solution bearing pH of 7.60 is preferred. To authenticate the predicted outcomes shown in Table 7, a laboratory test followed the process variables condition of solution-9 was executed. The experimental outcome produced the removal efficiencies of phenol, 4-CP and COD equaled to 84.6 ± 4.0, 95.5 ± 2.6 and 89.3 ± 3.1 %, respectively, which were in accordance with the predicted results (Table 7).
Conclusions
As wastewater is typically laden with mixture of phenolic compounds, research in the simultaneously adsorption of phenolic compounds is crucial to defuse their deleterious effects upon discharging to the natural water bodies. The CCD of RSM generated models could precisely predict the percentage removals of phenol, 4-CP and COD which signified the models reliability. The experimented process variables namely, SAC dosage and pH, did not conspicuously interact amidst the removal of phenol via SAC adsorption. Furthermore, only SAC dosage played the role to kindle the phenol adsorption proportionally to its quantity. However, the interaction of process variables was observed at their coded levels from 0 to +1 coded units whilst the 4-CP was adsorbed by SAC. The interaction within this range showed that the increase of pH had impoverished the 4-CP adsorption though raising the SAC dosage could partially offset the unfavorable effect of pH. As COD in the binary solution was virtually due to the presence of phenol and 4-CP, the process variables influenced the COD adsorption manifested the intermediary interaction characteristics of these phenolic compounds. The adsorption capacity of SAC revealed that 90 and 100 % of phenol and 4-CP, respectively, could be removed from the binary solution at the SAC dosage of 3.5 g/L and pH of lower than 9.00. The CCD program predicted nine possible optimum process variable conditions for the maximum removal of phenol, 4-CP and COD, in which SAC dosage all remained constant at 3.5 g/L. In view of the environmental gains, optimum condition at pH 7.60 was chosen which resulted in the percentage removals of phenol, 4-CP and COD correspondingly at 85.4 % (73.1 mg/g), 96.2 % (82.6 mg/g) and 90.9 % (78.0 mg/g); and was subsequently confirmed by the experimental results.
References
APHA, AWWA, WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association (APHA), Washington DC
Bas D, Boyaci IH (2007) Modeling and optimization I: usability of response surface methodology. J Food Eng 78(3):836–845
Bashir MJK, Abdul Aziz H, Yusoff MS, Adlan MN (2010) Application of response surface methodology (RSM) for optimization of ammoniacal nitrogen removal from semi-aerobic landfill leachate using ion exchange resin. Desalination 254(1–3):154–161
Bashir MJK, Abdul Aziz H, Yusoff MS (2011) New sequential treatment for mature landfill leachate by cationic/anionic and anionic/cationic process: optimization and comparative study. J Hazard Mater 186(1):92–102
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3(4):773–790
Busca G, Berardinelli S, Resini C, Arrighi L (2008) Technologies for the removal of phenol from fluid streams: a short review of recent developments. J Hazard Mater 160(2–3):265–288
Dabrowski A, Podkoscielny P, Hubicki Z, Barczak M (2005) Adsorption of phenolic compounds by activated carbon: a critical review. Chemosphere 58(8):1049–1070
Deng X, Yue Y, Gao Z (1997) Preparation and characterization of active carbon adsorbents for wastewater treatment from elutrilithe. J Colloid Interface Sci 192(2):475–480
Dotto GL, Costa JAV, Pinto LAA (2013) Kinetic studies on the biosorption of phenol by nanoparticles from Spirulina sp. LEB 18. J Environ Chem Eng 1(4):1137–1143
Environmental Quality (Sewage and Industrial Effluents) Regulations (2000) Malaysia sewage and industrial effluent discharge standards. Malaysia’s Environmental Law, Environmental Quality Act, 1974
Foo KY, Hameed BH (2009) Recent developments in the preparation and regeneration of activated carbons by microwaves. Adv Colloid Interface Sci 149:19–27
Ghafari S, Abdul Aziz H, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation-flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163(2–3):650–656
Gupta VK, Nayak A, Agarwal S, Tyagi I (2014) Potential of activated carbon from waste rubber tire for the adsorption of phenolics: effect of pre-treatment conditions. J Colloid Interface Sci 417:420–430
Guven G, Perendeci A, Tanyolac A (2008) Electrochemical treatment of deproteinated whey wastewater and optimization of treatment conditions with response surface methodology. J Hazard Mater 157(1):69–78
Hamdaoui O, Naffrechoux E, Tifouti L, Petrier C (2003) Effects of ultrasound on adsorption-desorption of p-chlorophenol on granular activated carbon. Ultrason Sonochem 10(2):109–114
Ivancev-Tumbas I, Hobby R, Kuchle B, Panglisch S, Gimbel R (2008) p-Nitrophenol removal by combination of powdered activated carbon adsorption and ultrafiltration—comparison of different operational modes. Water Res 42(15):4117–4124
Joglekar AM, May AT (1987) Product excellence through design of experiments. Cereal Foods World 32:857–868
Khan AR, Al-Bahri TA, Al-Haddad A (1997) Adsorption of phenol based organic pollutants on activated carbon from multi-component dilute aqueous solutions. Water Res 31(8):2102–2112
Kim HJ, Moon H, Park HC (1985) Multicomponent adsorption equilibria of phenols on activated carbon. Korean J Chem Eng 2:181–187
Kumar S, Zafar M, Prajapati JK, Kumar S, Kannepalli S (2011) Modeling studies on simultaneous adsorption of phenol and resorcinol onto granular activated carbon from simulated aqueous solution. J Hazard Mater 185:287–294
Leitao A, Serrao R (2005) Adsorption of phenolic compounds from water on activated carbon: prediction of multicomponent equilibrium isotherms using single component data. Adsorption 11:167–179
Lim JW, Ng SL, Khor SM, Seng CE (2012) Inhibitory effect of 2,4-dichlorophenol on nitrogen removal in a sequencing batch reactor. Korean J Chem Eng 29(7):886–890
Lim JW, Gan HM, Seng CE (2013a) Bioremediation of wastewaters containing various phenolic compounds by phenol-acclimated activated sludge. Desalin Water Treat 51(37–39):7018–7024
Lim JW, Tan JZ, Seng CE (2013b) Performance of phenol-acclimated activated sludge in the presence of various phenolic compounds. Appl Water Sci 3(2):515–525
Montgomery DC (2008) Design and Analysis of Experiments, 7th edn. Wiley, New York
Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response surface methodology, process and product optimization using designed experiments, 3rd edn. John Wiley and Sons, Inc., Hoboken
Nouri S (2002) Comparison of adsorption capacity of p-cresol & p-nitrophenol by activated carbon in single and double solute. Adsorption 8:215–223
Quintelas C, Sousa E, Silva F, Neto S, Tavares T (2006) Competitive biosorption of ortho-cresol, phenol, chlorophenol and chromium(VI) from aqueous solution by a bacterial biofilm supported on granular activated carbon. Process Biochem 41(9):2087–2091
Rawajfih Z, Nsour N (2006) Characteristics of phenol and chlorinated phenols sorption onto surfactant-modified bentonite. J Colloid Interface Sci 298(1):39–49
Sahinkaya E, Dilek FB (2007) Effect of feeding time on the performance of a sequencing batch reactor treating a mixture of 4-CP and 2,4-DCP. J Environ Manage 83(4):427–436
Shao Y, Zhang H, Yan Y (2013) Adsorption dynamics of p-nitrophenol in structured fixed bed with microfibrous entrapped activated carbon. Chem Eng J 225:481–488
Shehzad A, Bashir MJK, Sethupathi S, Lim JW (2015) An overview of heavily polluted landfill leachate treatment using food waste as an alternative and renewable source of activated carbon. Process Saf Environ Prot 98:309–318
Shehzad A, Bashir MJK, Sethupathi S, Lim JW (2016) An insight into the remediation of highly contaminated landfill leachate using sea mango based activated bio-char: Optimization, isothermal and kinetic studies. Desalin Water Treat. doi:10.1080/19443994.2015.1130660
Sunil K, Jayant K (2013) Adsorption for phenol removal—a review. Int J Sci Eng Res 1:88–96
Tarighian A, Hill G, Headley J, Pedras S (2003) Enhancement of 4-chlorophenol biodegradation using glucose. Clean Tech Environ Policy 5(1):61–65
Tobajas M, Monsalvo VM, Mohedano AV, Rodriguez JJ (2012) Enhancement of cometabolic biodegradation of 4-chlorophenol induced with phenol and glucose as carbon sources by Comamonastestosteroni. J Environ Manage 95:S116–S121
Toth A, Torocsik A, Tombacz E, Laszlo K (2012) Competitive adsorption of phenol and 3-chlorophenol on purified MWCNTs. J Colloid Interface Sci 387(1):244–249
Valo RJ, Häggblom MM, Salkinoja-Salonen MS (1990) Bioremediation of chlorophenol containing simulated ground water by immobilized bacteria. Water Res 24:253–258
WHO (1989) Chlorophenols other than pentachlorophenol. Environmental health criteria 93. World Health Organization, Geneva
WHO (1994) Phenols Standards. International Programme on Chemical Safety (IPCS), INCHEM, Environmental Health Criteria 161. World Health Organization, Geneva
Xiong L, Zheng L, Xu J, Zheng D, Li J, Li X, Sun J, Liu Q, Niu L, Yang S, Xia J (2011) Photocatalytic degradation of phenol with mesoporous TiO2-xBx. Environ Chem Lett 9(2):251–257
Yang G, Chen H, Qin H, Feng Y (2014) Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl Surf Sci 293:299–305
Zareen K, Anjaneyulu Y (2005) Influence of soil components on adsorption-desorption of hazardous organic-development of low cost technology for reclamation of hazardous waste dumpsites. J Hazard Mater 118(1–3):161–169
Acknowledgments
The kind financial support from MyBrain 15 (MyMaster) programme is gratefully acknowledged by authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Leong, KY., See, S., Lim, JW. et al. Effect of process variables interaction on simultaneous adsorption of phenol and 4-chlorophenol: statistical modeling and optimization using RSM. Appl Water Sci 7, 2009–2020 (2017). https://doi.org/10.1007/s13201-016-0381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0381-8