Abstract
Soil and water quality determines the health of an aquatic ecosystem. Rajakhali Canal, a tributary of Karnaphuli River estuary, flowing through Chittagong City (the commercial capital of Bangladesh) receives a huge amount of domestic and industrial wastes and sewages. Monitoring the environmental status of Karnaphuli River and its tributaries is very important for their ecological and economical services provided to city areas. This study evaluated some environmental characteristics of water and soil in the Rajakhali Canal as it affected the environment, and ultimately the life and human beings of Chittagong City. The mean concentrations of physico-chemical parameters were pH (8.5), DO (0.1 mg/L), TA (47.6 mg/L), TDS (631.8 mg/L), TSS (280 mg/L), SO4-S (2.3 mg/L), NH3 (1.1 mg/L), NO3-N (0.2 mg/L) and PO4-P (0.1 mg/L) in the dry season. During the rainy season, the mean concentrations of physico-chemical parameters were pH (7.01), DO (0.55 mg/L), TA (65.9 mg/L), TDS (653.6 mg/L), TSS (300.3 mg/L), SO4-S (1 mg/L), NH3 − (0.6 mg/L), (NO3-N (0.3 mg/L) and PO4-P (0.5 mg/L) in water. In case of soil, the mean concentration of physico-chemical parameters in dry and rainy seasons was represented respectively as follows: pH (6.8), OM (4.5 %), sand (71.7 %), silt (3.1 %), clay (25.2 %), organic nitrogen (45.4 ppm) and phosphorus (9.6 ppm); and pH (6.7), OM (4.5 %), sand (74.4 %), silt (2.4 %), clay (23.2 %), organic nitrogen (35.3 ppm) and phosphorus (7.6 ppm). The result revealed that water and soil quality of this canal became deteriorated and that the total environment of the water body became polluted due to the anthropogenic activities such as industrial, domestic and irrigation effluents. Statistical analyses also supported that water and soil parameters were strongly correlated (1-tailed 0.05 level and 0.01 level significant) with each other at all stations during all seasons. The result of this study will be useful for management and planning for water quality monitoring in this estuary. To protect this vital estuarine region, the government agencies, private agencies and scientists should work with proper attention.
Similar content being viewed by others
Introduction
Water is the most vital element among the natural resources and is crucial for the survival of all living organisms including humans, for food production and economic development (Shiklomanov 1993; Pahl-Wostl et al. 2008). Today, nearly 40 % of the world’s food supply is grown under irrigation, and a wide variety of industrial processes depend on water (BCAS 2000). Moreover, in Bangladesh, the environment, economic growth and developments are all highly influenced by the quality and quantity of surface and groundwater. The seasonal availability of surface and groundwater depends on the monsoon climate and topography of the country (Alam 2009). In terms of quality, the surface water of the country is vulnerable to pollution from untreated industrial effluents and municipal wastewater, runoff from chemical fertilizers and pesticides, and oil and lube spillage in the coastal area from the operation of sea and river ports (Hossain 2001). Water quality also depends on effluent types and discharge quantity from different type of industries, types of agrochemicals used in agriculture and seasonal water flow and dilution capability by the river system (DHV 1998).
Sewage may be defined as the products of municipal drainage system, containing domestic wastes with or without the addition of discharges from industry (including highly acid and alkaline wastes, oil greases, some heavy metals effluents, etc.) storm water and surface runoff (Grant et al. 2005). Classes of sewage include sanitary, commercial, industrial, agricultural and surface runoff. The wastewater from residences and institutions, washing water, food preparation wastes, laundry wastes and other waste products of normal living are classed as domestic or sanitary sewage (Kennish 1996). Liquid-carried wastes from stores and service establishments serving the immediate community, termed commercial wastes, are included in the sanitary or domestic sewage category if their characteristics are similar to household flows. Wastes that result from an industrial process or the production or manufacture of goods are classed as industrial wastewater (Kennish 1996). Their flows and strengths are usually more varied, intense and concentrated than those of sanitary sewage. Surface runoff, also known as storm flow or overland flow, is that portion of precipitation that runs rapidly over the ground surface to a defined channel.
Precipitation absorbs gases and particulates from the atmosphere, dissolves and leaches materials from vegetation and soil, suspends matter from the land, washes spills and debris from urban streets and highways and carries all these pollutants as wastes in its flow to a collection point (Luo et al. 2008). Under natural conditions, only when the concerning ecosystems are not balanced sewage causes various sorts of problems. Sewage contains a huge amount of phosphorus (P) and nitrogen (N) causing eutrophication and fatal consequents to the surrounding environment (Landolt and Kandeler 1987).
The worldwide water and soil quality deterioration are primarily contributable to growing human populations and economic development, particularly elevating nutrients leading to eutrophication and pollution in the aquatic environment (Nriagu and Pacyna 1988; Peierls et al. 1998; Holloway et al. 1998; Li et al. 2009; Pekey et al. 2004, Venkatramanan et al. 2013a, 2014a, b). The natural sources include volcanism, bedrock erosion, atmospheric transport and the release from plants (Khan et al. 1996; Pekey et al. 2004) and anthropogenic activities; particularly, mining and mineral processing have dominant influences of primary water and soil quality parameters.
There are rather few studies published in recent years on pollution sources in Karnaphuli River (Hossain 1992, 2001). In this existing scenario, this research aims at determining the pollution sources of Rajakhali Canal, which is adversely impacted by industries together with landfill leachate and municipal sewage effluents. This study evaluates the seasonal variation, source and contamination levels of water and soil quality.
Materials and methods
Study area settings
Bangladesh extending from 20°30′ to 26°N and 88° to 90°50′E, a small riverine country of 145,000 km2 (Alam 1994), lies in the north-east part of the South Asian sub-continent. The country is bounded by India in its north, east and west and for a small stretch in the southeast. It has a common border with Myanmar in the south-east and the Bay of Bengal is located in the south. The Ganges and the Brahmaputra Delta is situated at the apex of the Bay of Bengal. The country is crisscrossed by numerous large and small rivers. Most of the big rivers originate outside the country, i.e., India, Nepal and Myanmar. These rivers have a strong influence on the aquatic environment in varying degrees and time sequence, water quality, circulation, nature of bottom and the inhabitants of that environment (Banglapedia 2004).
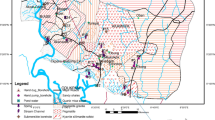
The Karnafully River Estuary is a strong tidal current type. The huge pollution load is reduced due to its dilution capacity (Mahmood et al. 1992). The Karnafully River is the largest and most important river in Chittagong. The Rajakhali Canal (Fig. 1) is situated near the Karnafully Shah Amanat bridge and adjacent to the leather storage of the Chaktai area. It is in the east side of the Chaktai Canal. The mixing portion of this canal is very closely connected with the Chaktai Canal. The Rajakhali is the largest canal flowing through Chittagong Town and has a total length of about 5.57 km. It starts from Chawlk Bazar commercial area and falls into the Karnafully River estuary through Chawlk Bazar, Baddarhat, Rahattarpool, Kalamia Bazar, Boro-gorosthan, Beribad Area, Oyazipara, Rajakhali and Chaktai. The upstream end point, its top width and depth are about 5.85 and 1.11 m, respectively. At the downstream end point of the canal with the Karnafully River, its top width and depth are about 15.35 and 4.54 m, respectively. Nevertheless, it is the major sewage drainage canal of the city and carries at least 18 % wastes and sewage of the city (Ahmed 1995).
Sample collection and analysis
The investigation was carried out in the municipal sewage drainage canal named Rajakhali of Chittagong metropolitan area. A total of 8 samples were collected in this canal system. (Table 1). The water and soil samples were collected from dry (February) and rainy (December) seasons in 2011. For water, soil and sewage samples were collected in glass bottles, polythene bag and BOD bottles. Sewage samples were collected for determining DO. They were placed in a wooden box to avoid direct sunlight, and sewage, water and soil samples were transported to the laboratory for further analysis. All samples were kept at 4 °C in a refrigerator before analysis (WHO and UNEP 1990). The water temperature was measured with a mercury thermometer; water pH by a pen pH meter, dissolved oxygen by Winkler method (Barnes 1959) and salinity of water by a hand refractometer in field. The average monthly rainfall data are shown in Fig. 2. Total dissolved solids (TDS) and total suspended solids (TSS) were measured by the method described in APHA (1995), alkalinity was measured by a method described by Suess (1982), total hardness was measured by a standard method (APHA 1995) and carbon dioxide was measured by the titrimetric method (De Cura et al. 1996). Unionized ammonia was determined by the phenol hypochloride method (APHA 1995), and nitrite-nitrogen (NO2-N), phosphate-phosphorous (PO4-P) and sulfate-sulfur (SO4-S) were determined by the ultraviolet spectrophotometric method (APHA 1995). Soil pH of water was determined by digital soil pH meter, soil texture was analyzed by the formula described by Boyd and Tucker (1992), soil salinity was measured by the formula described by Richard (1954) and soil alkalinity method described by Boyd and Pippopinyo (1994). The organic matter of the soil was determined by a method described by Jackson (1958). On the other hand, total nitrogen (N2) or organic nitrogen (N2) was determined by micro Kjeldahl digestion and distillation procedure (Strickland and Parsons 1968). Standards and blanks were run frequently to check the accuracy of the procedures. Care was taken to avoid handling errors. Few samples were repeated to check concordant readings. All the chemicals used were of analytical grade and procured from Merck. The accuracy of analytical experiments was determined by calculating the ionic balance error, which was generally within ±5 %. The accuracy of the soil analytical method was examined by the standard reference material MAG-1 (marine mud from the United States Geological Survey).
Results and discussion
Seasonal variation of water parameters
Throughout the study period, the maximum concentration of physico-chemical parameters in water were observed in the dry season except DO, TH, NO2-N and PO4-P. In the present study, water temperature of the studied area varied from 27 to 33 °C during the dry season and from 26 to 29.25 °C during the rainy season (Fig. 3). Water pH was from 8.1 to 8.8 during the dry season and from 6.5 to 7.7 during the rainy season (Fig. 4). The maximum value of water temperature was 33 °C in Station 7 during the dry season and the minimum value was 26 °C in Stations 1, 2 and 4 during the rainy season. The difference between maximum and minimum water temperature was higher in the present study than in the previous year observed by Hossain (1992). Water temperature increases in a stream, and DO decreases due to the lower solubility of oxygen at higher temperatures (Klein 1962). Remani et al. (1983) and Chandran and Ramamoorthi (1984) mentioned that the temperature fluctuation was a seasonal phenomenon resulting from the monsoonal effect. The highest value of pH was recorded as 8.8 in Stations 7 and 8 during the dry season. On the other hand, the lowest value was 6.5 in Stations 1 and 2 during the rainy season, respectively. The value of pH in the discharged area showed a slightly acidic nature. This study showed that the water pH of eight sampling stations was changed from 6.5 to 8.8. This indicates that the effluent of industrial and municipal waste materials had a significant role in increasing or decreasing pH of the adjacent water body where the waste materials were dumped (Moore 1972; Bhouyan 1979; Mahmood et al. 1992).
During the dry season, dissolved oxygen varied from 0 to 9.7 mg/L (Fig. 5), but during rainy season from 20 to 56 mg/L. The salinity values varied from 0.2 to 1 ppt in both seasons(Fig. 6). The low DO of effluent and effluent discharged area might be due to high content of total suspended solids and total dissolved solids, which utilized the significant amount of DO for bio-chemical degradation. The higher value of DO may be due to large amount of wastes discharged by the sewage system in both seasons (Hossain 1992). During the dry season, salinity was found to be higher than that of the rainy season. This exhibits the large amount of fresh water discharge from monsoonal rainfall. Total hardness of the eight sampling stations fluctuated between 36.30 and 88.9 mg/L (Fig. 7). The highest value of 88.9 mg/L was found in Station 4 during the rainy season. On the other hand, the lowest value was 36.30 mg/L in Station 6 during the dry season. This may be due to the presence of different types of total salts discharged from industries and domestic sewage garbage (Sapon 1993; Hossain 1992). Total dissolved solids of the eight sampling stations fluctuated between 334 and 951 mg/L (Fig. 8). The cause of the highest and lowest values of TDS in the discharged area is due to proper and improper dilution of effluents and wastes. Moore (1972) also stated that TDS in water contained chiefly ammonia, nitrite, nitrate, phosphate, alkali, etc. The highest value of alkalinity (Fig. 9) was found to be 270 mg/L in Station 7 during the dry season and the lowest value was 78 mg/L in Station 6 during the rainy season due to municipal waste disposal and oil pollution (Thomas and Lynch 1960). This similar observation was made by Mahmood et al. (1992) and Paul (1981) who reported that oil pollution in the Karnaphuli River was responsible for increasing the total alkalinity of this river and its tributaries. The high and low value of free carbon dioxide ranged from 18.78 to 87.12 mg/L in rainy and dry seasons, respectively (Fig. 10). According to the Department of Environment (DOE 1993), the standard value of CO2 in the water body is 6 mg/L. Carbon dioxide is a normal component of all natural waters. It dissolves in water in varying amounts, and the dissolution depends on the temperature, pressure and mineral content of the water. Polluted waters acquire carbon dioxide by the biological oxidation of organic matter (Rashid 1996). Sulfate-sulfur (SO4-S, varied from 0.86 to 3.60 mg/L in rainy and dry seasons, respectively (Fig. 11). Sulfate-sulfur was mostly related with soil and water pH and other wastes. After submergence, the pH of acid sulfate soils gradually increased due to reduction and inactivation of SO4-S through microbial activity in both seasons (Smith 1998). This reduction rate depends on the presence of sulfur-reducing bacteria, the pH of the system, energy source (such as organic matter), etc. (Paul 1981). As SO4 is reduced, the pH rises. However, the change is not instantaneous and several weeks of submergence may be needed to increase the pH. From statistical analysis, it is found that water temperature is positively correlated with pH, salinity, alkalinity and SO4-S during the present study and negatively correlated with total hardness and DO (0.01 level). It is also supported that all the parameters are strongly correlated with each other in all stations and seasons (Table 2). Nitrite-nitrogen (NO2-N, Fig. 12) ranged from 0.22 to 0.40 mg/L in the dry and rainy seasons, respectively. The concentration of nitrite was generally found to be much lower than that of the nitrate. It did not show any regular pattern of seasonal variation like nitrate. Although nitrite-nitrogen had a seasonal variation in the present investigation, an abrupt rise in concentration was found in both periods due to industrial and municipal wastes (Nair and Gonapathi 1983; Verlencar 1987). Phosphate-phosphorous (PO4-P) of the eight sampling stations fluctuated between 0.045 and 0.915 mg/L (Fig. 13). The higher value might be due to the addition of effluent, rich in phosphate content, and bio-degradation of human wastes and organic matter. On the other hand, it was also observed that DO was completely zero at stations with anaerobic condition. When this sewage was discharged into river water, it caused different types of water-borne diseases, because of bearing pathogenic bacteria. The concentrations of these parameters were from a common source of origin and might be due to the high amount of dissolved ions in this canal.
Seasonal variation of soil parameters
A maximum concentration of physico-chemical parameters were observed in the dry season compared to the rainy season except for sand. Soil pH was recorded as 7.0 at Stations 1 and 2 during the rainy season, and the minimum value observed was 6.7 at Stations 1, 2 and 7 in the dry season (Fig. 14). With regard to the relationship of pH to percent base saturation, the pH is normally highest in hydrous oxide clay materials, intermediate in kaolinite and humus, and lowest in minerals. Obviously, the type of clay definitely affects the pH base saturation percentage relationship. The higher the exchange capacity of the sediment, the greater is the buffer capacity (Farhadinejad et al. 2014), but other factors are equal. Thus, the higher the clay and organic matter contents, the more is the lime required for a given change in the sediment pH (Barua and Zamal 2011). Organic matter percentage ranged from 2.18 to 5.77 % during the rainy and dry seasons, respectively (Fig. 15). The fine texture of the soil in this environment also represents the accumulation of organic matter. This resulted from very low rates of decomposition due to consistent anaerobic condition created by the almost permanent saturation with water (Farhadinejad et al. 2014). High organic matter content poses threat to the depletion of DO in water (Boyd 1990).
Sand particles fluctuated from 67.88 to 81.37 % (Fig. 16). So this may be due to the rainfall effect, flocculation and coagulation process. The highest value of 81.37 was found in Station 4 during the rainy season, and the lowest value was 67.88 in Station 6 during the dry season. The sands group includes all soils in which the sand separates make up at least 70 % and the clay separate 15 % or class of the material by weight. The properties of such soils are therefore characteristically those of sand in contrast to stickier nature of clays. Clay separate (Fig. 17) ranged from 17.59 to 28.71 % in the rainy and dry seasons, respectively. To be designated clayey, the soil must contain at least 35 % of clay separate and in most cases not less than 40 %. In such soils, the characteristics of the clay separate are distinctly dominant, and the class names are clay and silt clay. Sandy clays may contain more sand than clay. The percentage of silt (Fig. 18) varied from 1.04 to 4.36 % in both seasons. There was a considerable variation in the soil parameters not only among the canals, but also between spots of the same canal. This should emphasize that the canal bottoms were uneven in soil quality. This variation could have been created by sedimentation processes during the past sewage swamp system (Rahman 1992; Barua and Zamal 2011; Venkatramanan et al. 2013a, b; Hossain et al. 2014). Scientific studies have shown that the heavy metal concentration is controlled mainly by the textural composition of the sample, i.e., fine-grained sediments register higher concentrations of trace metals than sand-dominant sediments (Hossain et al. 2014; Dar 2014; Venkatramanan et al. 2014a, b; Machender et al. 2014; Rajganapathi et al. 2013). This information has wide-ranging implications from the environmental perspective as these contaminants get dispersed and find their way into the food web of the aquatic ecosystems.
In the present investigation, experimental soils were rather poor in total nitrogen (organic nitrogen) (Fig. 19) in comparison to the organic matter content. The probable high C/N ratios may inhibit the decomposition of organic matter. This shows the characteristics of the peat layers. A high organic matter poor in nitrogen is also conducive to reduction processes. These concentrations may be reflected from anthropogenic influences. Phosphorus concentration was not very low, being above 24 ppm (Fig. 20). In acid sulfate soils, low available phosphorous resulted because of low solubility in acid reaction, insolubilization or fixation by Fe, Al and Mn and low release from organic matter. In some acid sulfate fish ponds, the addition of phosphate fertilizer has been found to be ineffective because of the fixation of added phosphorous in the form of irreversible iron and aluminum phosphate (Poernomo and Singh 1982). In alkaline condition, on the other hand, colloidal materials in mud and organic matter may inactivate phosphorus (Watts 1980). From statistical analysis, it was found that soil pH was positively correlated with organic nitrogen (0.01 level) and organic matter significantly correlated with soil texture (0.05 level) (Table 3).
Conclusion
The present research showed that the statistical analysis of physico-chemical parameters in water and soil was helpful to elucidate the pollution sources of the Rajakhali Canal. The physico-chemical parameters in water were listed in the descending order of concentration: TDS > TSS > SO4-S > NH3 > NO3-N > PO4-P in the dry season; TDS > TSS > SO4-S > NH3 > PO4-P > NO3-N in the rainy season. The interpretation of soil analytical data is denoted as follows: sand > clay > silt > OM > organic nitrogen > phosphorous in both seasons. A maximum concentration of physico-chemical parameters of water and soil was observed in the dry season rather than rainy season, except for DO, TH, NO2-N, PO4-P and sand in water and sediment, respectively. The higher concentration of nutrients in water and soil suggested that the effluents were derived from industrial, domestic and irrigation fields. Statistical analysis also supported that water and soil parameters were strongly correlated (1-tailed 0.05 level and 0.01 level significant) each other for controlling the Rajakhali Canal system. The present study on water and soil revealed that the surrounding areas were polluted and harmful for eco-aquatic life. To protect this vital estuarine region, the government agencies, private agencies and scientists should work together with proper attention. Further investigations are in progress to determine the level and sources of contamination using another technique.
References
Ahmed S (1995). Comparative study of Municipal Sewage, Chittagong. M.Sc. Thesis, Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 58
Alam MM (1994). Study on the heavy metal concentrations in estuarine water and shellfish of the Karnafully River estuary, Chittagong. M.Sc. Thesis (unpublished), Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 44
Alam GJ (2009) Environmental pollution of Bangladesh—it’s effect and control. Pulp Paper 51:13–17
American Public Health Association (APHA). (1995). Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association, and Water Pollution Control Federation. 19th edition, Washington, D.C
Banglapedia (2004) Asiatic Society of Bangladesh. http://www.banglapedia.org/HT/K_0121.htm
Barnes H (1959) Apparatus and methods of oceanography (Chemical), vol 1. London and New York Academy Press. pp 178–183
Barua P, Zamal H (2011). Nutrient dynamics for coastal aquaculture of Bangladesh. Lap Lambert Publishing, p 219
BCAS (2000) Pollution Study. Management of Aquatic Ecosystem through Community Husbandry (MACH), Dhaka, p 102
Bhouyan AM (1979) Effect of industrial pollution on the biology of the Karnafully River. M. Phil. Thesis, University of Chittagong, Bangladesh, p 164
Boyd CE (1990) Water quality in ponds for aquaculture. Alabama Agricultural Experiment Station, Auburn University, Alabama, p 482
Boyd CE, Pippopinyo S (1994) Factors affecting respiration in dry pond bottom soils. Aquaculture 120:283–293
Boyd CE, Tucker CS (1992) Water quality and pond soil analyses for aquaculture. Alabama Agricultural Experiment Station, Auburn University, Alabama, p 183
Chandran R, Ramamoorthi K (1984) Hydrobiological studies in the gradient zone of the Vellar Estuary: 1. Physico-chemical parameters. Mahasagar 17(2):69–77
Dar MA (2014) Distribution patterns of some heavy metals in the surface sediment fractions at northern Safaga Bay, Red Sea, Egypt. Arab J Geosci 7(1):55–67
De Cura B, Escaribano MI, Zamorano JP, Merodrio C (1996) High CO2 treatment delays postharvest changes in RuBP Case ad Polygalacturonase-related protein in cherimoya pee. J Am Soc Hortic Sci 121:735–739
Department of Environment (1993) Institutional arrangement of coastal zone management report prepared by Bangladesh centre for advanced studies, p 45
DHV (1998) Southwest area water resources development project. Feasibility study, final reports (vol 2 and 3) to Bangladesh water development board, Government of Bangladesh
Farhadinejad T, Khakzad A, Jafari M, Shoaee Z, Khosrotehrani K, Nobari R, Shahrokhi V (2014) The study of environmental effects of chemical fertilizers and domestic sewage on water quality of Taft region, Central Iran. Arab J Geosci 7(1):221–229
Grant N, Moodie, Weedon C (2005) Sewage solutions. Powys. CAT, p 190
Holloway JM, Dahlgren RA, Hansen B, Casey WH (1998) Contribution of bedrock nitrogen to high nitrate concentrations in stream water. Nature 395:785–788
Hossain T (1992) Study on the Environment Impact Assessment (EIA) of the effluent discharged by the Chittagong Urea Fertilizer Limited (CUFL) on the Karnafully River estuary, M.Sc. Thesis, Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 55
Hossain M (2001) Biological aspects of the coastal and marine environment of Bangladesh. Ocean Coast Manag 44(3):261–282
Hossain MB, Marshall DJ, Venkatramanan S (2014) Sediment granulometry and organic matter content in the intertidal zone of the Sungai Brunei estuarine system, Northwest coast of Borneo. Carpath J Earth Environ Sci 9:231–239
Jackson ML (1958) Soil chemical analysis. Prentice-Hall Inc. Englewood Cliffs, p 231
Kennish MJ (1996) Practical handbook of estuarine and marine pollution, vol 10. CRC press, Taylor and Francis Group, pp 544
Khan YSA, Ahammod MS, Hossain MS (1996) Sewage pollution in Chittagong Metropolitan Area, Bangladesh. Oriental Geographer 40:69–77
Klein L (1962) River pollution 2. Causes and Effects. Butter Worths and Co. Ltd., London, p 206
Landolt E, Kandeler R (1987) Biosystematic Investigations in the Family of Duckweeds (Lemnaceae): The Family of Lemnaceae-a Monographic Study. Phytochemistry, physiology, application, bibliography. Geobotanisches Institut der ETH
Li Z, Fang Y, Zeng G, Li J, Zhang Q, Yuan Q, Wang Y, Ye F (2009) Temporal and spatial characteristics of surface water quality by an improved universal pollution index in red soil hilly region of South China: a case study in Liuyanghe River watershed. Environ Geol 58:101–107
Luo XJ, Chen SJ, Ni HG, Yu M, Mai BX (2008) Tracing sewage pollution in the Pearl River Delta and its adjacent coastal area of South China Sea using linear alkylbenzenes (LABs). Mar Pollut Bull 56(1):158–162
Machender G, Dhakate R, Rao SM, Rao BM, Prasanna L (2014) Heavy metal contamination in sediments of Balanagar industrial area, Hyderabad, Andhra Pradesh, India. Arab J Geosci 7(2):513–525
Mahmood N, Chowdhury MSU, Hossain MM, Haider SMB, Chowdhury SR (1992) Review of the State of Environment relating to Marine Fisheries of Bangladesh. Country status report. BOBP (FAO) IMS. CU, p 85
Moore P (1972) Studies on the pollution of the Bhadra River Fisheries and BhadraVathi (Mysore State) with industrial effluents. Nat Int Sci Ind 22:132–160
Nair KVK, Gonapathi A (1983) Baseline Ecology of Edaeyur Sadras Estuarine system at Kalpakkam part-1. General hydrographic and chemical features. Mahassagar Bull Natl Inst Oceanogr 16:143–151
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace-metals. Nature 333:134–139
Pahl-Wostl C, Mostert E, Tàbara D (2008) The growing importance of social learning in water resources management and sustainability science. Ecol Soc 13(1):24
Paul S (1981). Effect of oil pollution upon planktonic organisms of the Karnafully River Estuary. M. Sc. Thesis, Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 48
Peierls BL, Caraco NF, Pace ML, Cole JJ (1998) Human influence on river nitrogen. Nature 350:386–387
Pekey H, Karaka D, Bakoglu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar Pollut Bull 49:809–818
Poernomo A, Singh VP (1982) Problems, field identification and practical solutions of acid sulfate soils for brackishwater fishponds. Proc. Semi. Fish. Pond Engg., South China Sea Program/Food and Agri. Orga., Surabaya, Indonesia, p 49–61
Rahman K (1992) Industrial pollution and control for sustainable development. Training Manual on Environmental Management in Bangladesh. DoE, Dhaka, pp 184–206
Rajganapathi VC, Jitheshkumar N, Sundararajan M, Bhat KH, Velusamy S (2013) Grain size analysis and characterization of sedimentary environment along Thiruchendur coast, Tamilnadu, India. Arab J Geosci 6(12):4717–4728
Rashid MMU (1996) Study on water quality and commercial Ichthyo Fauna of the Bankkhali River Estuary, M. Sc. Thesis, Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 58
Remani KN, Devi S, Venugopal K, Unnithan RV (1983) Indicator organisms of pollution in Cochin backwaters. Mahassagar Bull Natl Inst Oceanogr 16:199–207
Richard LA (1954) Diagnosis and improvement of saline and alkali soils. United States Department of Agriculture, Washington, D.C. USA: Hand book 60
Sapon JSM (1993) Environmental impact assessment (EIA) of the Municipal sewage discharge through Majhirghat canal on the Karnafully River water, Chittagong. M. Sc Thesis. Institute of Marine Sciences, University of Chittagong, Chittagong, Bangladesh, p 90
Shiklomanov IA (1993) World water resources. Water in Crisis. New York, Oxford
Smith WG (1998) The tidal area study. An Interim Report. FAO/UNDP fisheries resources survey system, Dhaka, p 58
Strickland JDH, Parsons TR (1968) A practical handbook of seawater analysis. Pigment analysis, Bulletin of fisheries research. Bd. Canada 167
Suess MJ (1982) Examination of water for pollution control, a Reference Handbook, Pergamon Press. England 3:276–300
Thomas JFJ, Lynch J (1960) Determination of carbonate alkalinity in natural waters. J Am Water Works Assoc 18:252–259
Venkatramanan S, Ramkumar T, Anithamary I (2013a) Distribution of grain size, clay mineralogy and organic matter of surface sediments from Tirumalairajanar Estuary, Tamilnadu, east coast of India. Arab J Geosci 6:1371–1380
Venkatramanan S, Ramkumar T, Anithamary I, Jonathan MP (2013b) Speciation of selected heavy metals geochemistry in surface sediments from Tirumalairajan river estuary, east coast of India. Environ Monit Assess 185:6563–6578
Venkatramanan S, Chung SY, Lee SY, Park N (2014a) Assessment of river water quality via environmentric multivariate statistical tools and water quality index: a case study of Nakdong river basin, Korea. Carpath J Earth Environ Sci 9:125–132
Venkatramanan S, Ramkumar T, Anithamary I, Vasudevan S (2014b) Heavy metal distribution in surface sediments of the Tirumalairajan river estuary and the surrounding coastal area, east coast of India. Arab J Geosci 7(1):123–130
Verlencar XN (1987) Distribution of nutrients in the coastal and estuarine water of Goa. Mahassagar Bull Natl Inst Oceanogr 20:205–215
Watts WA (1980) Late-Quaternary vegetation history at White Pond on the inner coastal plain of South Carolina. Quatern Res 13(2):187–199
WHO, UNEP (1990) Assessment of fresh water quality report on the results of the WHO/UNEP program on health related environmental monitoring, 1, p 32
Acknowledgments
The authors are grateful to Mr Sattar and the laboratory assistants who helped during sampling and in the IMSF laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. R. Islam and M. B. Hossain have contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Islam, M.R., Das, N.G., Barua, P. et al. Environmental assessment of water and soil contamination in Rajakhali Canal of Karnaphuli River (Bangladesh) impacted by anthropogenic influences: a preliminary case study. Appl Water Sci 7, 997–1010 (2017). https://doi.org/10.1007/s13201-015-0310-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0310-2