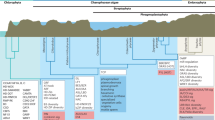
Abstract
Symbiosis may have played a far greater role in biological evolution than was previously thought. The symbiosis that made the colonization of land by plants possible was as a consequence of the development of arbuscular mycorrhizae. However, the present review draws attention to the role of lichens in assisting in this transition and to the phenomenon of lichenization. The recent discovery of lichen fossils in marine phosphorites in China and molecular clock estimates indicate that lichenized fungi were already present in Precambrian seas and, like contemporary species, played a role as pioneers in occupying new habitats. There is evidence that the holistic properties of associations between fungi and cyanobacteria and/or green algae facilitated the transition onto land and the subsequent colonization of terrestrial habitats. A key role in this process was played by poikilohydry. The algal components of delichenized fungi, along with lichens and photosynthetic aquatic organisms all contributed to the increase in atmospheric oxygen. Lichens, fungi and cyanobacteria settling on land were undoubtedly important in the formation of soils and thereafter enhancing their fertility. It is suggested that vascular and other green plants were able grow on these primitive soils that were stabilized by the growth of lichens, algae and cyanobacteria in a similar way to those which play a role in desert crusts at the present time.










Similar content being viewed by others
References
Alizadeh O (2011) Mycorrhizal symbiosis. Adv Stud Biol 3:273–281
Altermann S (2004) A second look at Letharia (Th.Fr.) Zahlbr. Bull Calif Lichen Soc 11(2):33–36
Antoine P-O, De Franceschi D, Flynn JJ, Nel A, Baby P, Benammi M, Calderón Y, Espurt N, Goswami A, Salas-Gismondi R (2006) Amber from western Amazonia reveals Neotropical diversity during the middle Miocene. Proc Natl Acad Sci 103(37):13595–13600
Aptroot A, Seaward MRD (1999) Annotated checklist of Hong Kong lichens. Trop Bryol 17:57–101
Becker B, Marin B (2009) Streptophyte algae and the origin of embryophytes. Ann Bot 103:999–1004
Beiggi S, Piercey-Normore MD (2007) Evolution of ITS ribosomal RNA secondary structures in fungal and algal symbionts of selected species of Cladonia sect Cladonia (Cladoniaceae, Ascomycotina). J Mol Evol 64:528–542
Beraldi-Campesi H (2013) Early life on land and the first terrestrial ecosystems. Ecol Process 2:1. doi:10.1186/2192-1709-2-1
Berbee ML, Taylor JW (2010) Dating the molecular clock in fungi: how close are we? Fungal Biol Rev 24:1–16
Bidartondo MI, Read DJ, Trappe JM, Merckx V, Ligrone R, Duckett JG (2011) The dawn of symbiosis between plants and fungi. Biol Lett 7:574–577
Blaha J, Baloch E, Grube G (2006) High photobiont diversity associated with the euryoecious lichen-forming ascomycete Lecanora rupicola (Lecanoraceae, Ascomycota). Bot J Linn Soc 88:283–293
Błaszkowski J (2004) Przeszłość, teraźniejszość i przyszłość klasyfikacji arbuskularnych grzybów mikoryzowych [The past, present and future of classification of arbuscular mycorrhizal fungi]. Kosmos 53:17–24
Bock R (2010) The give-and-take of DNA: horizontal gene transfer in plants. Trends Plant Sci 15:11–22. doi:10.1016/j.tplants.2009.10.001
Bonfante P, Genre A (2008) Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci 13:492–498
Brooks WR (2012) The importance of symbioses in biological systems. J Mar Sci Res Dev 2:1–3. doi:10.4172/2155-9910.1000e108
Büdel B, Vivas M, Lange OL (2013) Lichen species dominance and the resulting photosynthetic behavior of Sonoran Desert soil crust types (Baja California, Mexico). Ecol Process 2:6–15
Cairney JWG (2000) Evolution of mycorrhiza systems. Naturwissenschaften 87:467–475
Cardinale M, Puglia AM, Grube M (2006) Molecular analysis of lichen-associated bacterial communities. FEMS Microbiol Ecol 57:484–495
Carrapiço F (2010) How symbiogenic is evolution? Theory Biosci 129(2–3):135–139
Carrapiço F (2012) The symbiotic phenomenon in the evolutive context. In: Pombo O, Rahman S, Torres JM, Symon J (eds) Special sciences and the unity of science. Springer, Heidelberg, pp 113–119
Chaisson EJ (2001) Cosmic evolution: the rise of complexity in nature. Harvard University Press, Cambridge
Chu FJ, Seaward MRD, Hodgkiss IJ (2000) Effects of wave exposure and aspect on Hong Kong supralittoral lichens. Lichenologist 32:155–170
Church AH (1921) The lichen as transmigrant. J Bot 59(7–13):40–46
Clarke JT, Warnock RCM, Donoghue PCJ (2011) Establishing a time-scale for plant evolution. New Phytol 192:266–301
Cooper R (1953) The role of lichens in soil formation and plant succession. Ecology 34:805–807
Cordeiro LMC, Reis RA, Cruz LM, Stocker-Wörgötter E, Grube M, Iacomini M (2005) Photobionts of selected lichens from coastal vegetation of Brazil. FEMS Microbiol Ecol 54:381–390
Corning PA (1998) The synergism hypothesis. On the concept of synergy and its role in the evolution of complex systems. J Soc Evol Syst 21:133–172
Corning PA (2014) Systems theory and the role of synergy in the evolution of living systems. Syst Res Behav Sci 31:181–196
Czarnota P (2009) Symbiozy porostowe w świetle interakcji pomiędzy grzybami i fotobiontami [Lichen symbioses in the light of relationships between mycobionts and fotobionts]. Kosmos 58:229–248
de la Torre R, Sancho LG, Horneck G, de los Rios A, Wierzchos J, Olsson-Francis K, Cockell Ch S, Rettberg P, Berger T, de Vera J-PP, Ott S, Frías JM, Melendi PG, Lucas MM, Reina M, Pintado A, Demets R (2010) Survival of lichens and bacteria exposed to outer space conditions—results of the Lithopanspermia experiments. Icarus 208:736–748
de Vera J-P, Schulze-Makuch D, Khan A, Lorek A, Koncz A, Möhlmann D, Spohn T (2014) Adaptation of an Antarctic lichen to Martian niche conditions can occur within 34 days. Planet Space Sci 8:182–190
Delaux P-M, Séjalon-Delmas N, Bécard G, Ané J-M (2013) Evolution of the plant–microbe symbiotic ‘toolkit’. Trends Plant Sci 18:298–304
Delmail D, Grube M, Parrot D, Cook-Moreau J, Boustie J, Labrousse P, Tomasi S (2012) Halotolerance in lichens: symbiotic coalition against salt stress. In: Ahmad P, Azooz MM, Prasad MNV (eds) Ecophysiology and responses of plants under salt stress. Springer, Dordrecht, pp 115–148. doi:10.1007/978-1-4614-4747-4
Deschamps P (2014) Primary endosymbiosis: have cyanobacteria and Chlamydiae ever been roommates? Acta Soc Bot Pol 83:291–302
Dimijian GG (2000) Evolving together: the biology of symbiosis, part 1. Proc (Bayl Univ Med Cent) 13:217–226
Doebeli M, Knowlton N (1998) The evolution of interspecific mutualisms. Proc Natl Acad Sci 95:8676–8680
Domaschke S, Fernández-Mendoza F, García MA, Martín MP, Printzen C (2012) Low genetic diversity in Antarctic populations of the lichen-forming ascomycete Cetraria aculeata and its photobiont. Polar Res 31:1–13. doi:10.3402/polar.v31i0.17353
Edwards DS (1986) Aglaophyton major, a non-vascular land-plant from the Devonian Rhynie chert. Bot J Linn Soc 93:173–204
Eickmeier WG (1986) The correlation between high-temperature and desiccation tolerances in a poikilohydric desert plant. Can J Bot 64:611–617
Eriksson OE (2005) Ascomyceternas ursprung och evolution – Protolichenes-hypotesen. Svensk Mykol Tidsk 26:22–33
Farrar JF (1976) The lichen as an ecosystem: observation and experiment. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. Academic, London, pp 385–406
Federal Geographic Data Committee (FGDC) (2012) Coastal and marine ecological classification standard. FGDC-STD-18-2012. Reston, VA Federal Geographic Data Committee
Fletcher A (1973) The ecology of maritime (supralittoral) lichens on some rocky shores of Anglesey. Lichenologist 5:401–422
Gargas A, DePriest PT, Grube M, Tehler A (1995) Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 268:1492–1495
Garty J, Giele C, Krumbein WE (1982) On the occurrence of pyrite in a lichen-like inclusion in Eocene amber (Baltic). Palaeogeogr Palaeoclimatol Palaeoecol 39:139–147
Gasulla F, Herrero J, Esteban-Carrasco A, Ros-Barceló A, Barreno E, Zapata JM, Guéra A (2012) Photosynthesis in lichen: light reactions and protective mechanisms. In: Najafpour MM (ed) Advances in photosynthesis – fundamental aspects. InTech, Rijeka, pp 149–174. doi:10.5772/26204
Gilbert OL, Giavarini VJ (1997) The lichen vegetation of acid watercourses in England. Lichenologist 29:347–367
Giordano S, Colacino C, Spagnuolo V, Basile A, Esposito A, Castaldo-Cobianchi R (1993) Morphological adaptation to water uptake and transport in the poikilohydric moss Tortula ruralis. G Bot Ital 127:1123–1132
Gostinčar C, Muggia L, Grube M (2012) Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front Microbiol 3:390. doi:10.3389/fmicb.2012.00390
Gradstein FM, Ogg JG, Van Kranendonk M (2008) On the geologic time scale. Newsl Stratigr 43:5–13
Green TGA, Lange OL (1995) Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes. Ecophysiol Photosynth 100:319–341
Grube M, Hawksworth DL (2007) Trouble with lichen: the re-evaluation and re-interpretation of thallus form and fruit body types in the molecular era. Mycol Res 111:1116–1132
Gueidan C, Villaseñor CR, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119
Guzow-Krzeminska B (2006) Photobiont flexibility in the lichen Protoparmeliopsis muralis as revealed by ITS rDNA analyses. Lichenologist 38:469–476
Harańczyk H, Nowak P, Olech M (2013) Porosty antarktyczne – sposoby przetrwania w skrajnie nieprzyjaznym środowisku [Antarctic lichens – how to survive in an extremely hostile environment]. Kosmos 62:373–380
Harris PM (1996) Competitive equivalence in a community of lichens on rock. Oecologia 108:663–668
Hartard B, Cuntz M, Máguas C, Lakatos M (2009) Water isotopes in desiccating lichens. Planta 231:179–193
Hausrath EM, Neaman A, Brantley SL (2009) Elemental release rates from dissolving basalt and granite with and without organic ligands. Am J Sci 309:633–660
Hawksworth DL (1988) The variety of fungal–algal symbioses, their evolutionary significance, and the nature of lichens. Bot J Linn Soc 96:3–20
Hawksworth DL (2000) Freshwater and marine lichen-forming fungi. In: Hyde KD, Ho WH, Pointing SB (eds) Aquatic mycology across the millennium. Fungal Diversity 5:1–7. http://www.fungaldiversity.org/fdp/sfdp/FD_5_1-7.pdf
Heber U, Bilger W, Bligny R, Lange OL (2000) Phototolerance of lichens, mosses and higher plants in an alpine environment: analysis of photoreactions. Planta 211:770–780
Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB (2001) Molecular evidence for the early colonization of land by fungi and plants. Science 293:1129–1133
Heilmeier H, Durka W, Woitke M, Hartung W (2005) Ephemeral pools as stressful and isolated habitats for the endemic aquatic resurrection plant Chameagigas intrepidus. Phytocoenologia 35:449–468
Helms GWF (2003) Taxonomy and symbiosis in associations of Physciaceae and Trebouxia. Biologischen Fakultät der Georg-August Universität Göttingen. https://ediss.uni-goettingen.de/bitstream/handle/11858/00-1735-0000-0006-AE69-7/helms.pdf?sequence=1
Henskens FL, Green TGA, Wilkins A (2012) Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Ann Bot 110:555–563
Heywood A (2007) Ideologie polityczne. Wprowadzenie [Political ideologies. Introduction]. Wydawnictwo Naukowe PWN, Warszawa
Hill DJ (2009) Asymmetric co-evolution in the lichen symbiosis caused by a limited capacity for adaptation in the photobiont. Bot Rev 75:326–338
Högnabba F, Stenroos S, Thell A (2009) Phylogenetic relationships and evolution of photobiont associations in the Lobariaceae (Peltigerales, Lecanoromycetes, Ascomycota). Bibl Lichenol 100:157–187
Honegger R (1996) Morphogenesis. In: Nash TH (ed) Lichen biology. Cambridge University Press, Cambridge, pp 65–87
Honegger R (2001) The symbiotic phenotype of lichen-forming ascomycetes. In: Hock B (ed) The mycota. IX Fungal Associations. Springer, Heidelberg, pp 165–188
Honegger R, Edwards D, Axe L (2013) The earliest records of internally stratified cyanobacterial and algal lichens from the Lower Devonian of the Welsh Borderland. New Phytol 197:264–275, http://meetingorganizer.copernicus.org/EGU2012/EGU2012-2113.pdf
Humphreys CP, Franks PJ, Rees M, Bidartondo MI, Leake JR, Beerling DJ (2010) Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat Commun 1:103. doi:10.1038/ncomms1105
Jabłońska A (2012) Porosty z rodzaju Porpidia Körb. występujące w Polsce [The lichen genus Porpidia Körb. in Poland]. Monogr Bot 102:5–123
Jackson HB, Leavitt SD, Krebs T, Larry, Clair LLS (2005) Lichen flora of the eastern Mojave Desert: Blackrock Arizona, Mojave County, Arizona, USA. Evansia 22(1):30–38
Johnson LE (1991) A morally deep world: an essay on moral significance and environmental ethics. Cambridge University Press, Cambridge, pp 162–163
Jurina AL, Putiatina ON (2000) Revision of the genus Flabellifolium Stone, 1973 (group of Paleozoic plants with Ginkgo-like megaphylls) and the first findings of its Givetian members in Central Kazakhstan. Paleontol Zh 3:103–110
Kaasalainen U, Fewer DP, Jokela J, Wahlsten M, Sivonen K, Rikkinen J (2013) Lichen species identity and diversity of cyanobacterial toxins in symbiosis. New Phytol 198:647–651
Kappen L, Valladares F (2007) Opportunistic growth and desiccation tolerance: the ecological success of poikilohydric autotrophs. In: Pugnaire FI, Valladares F (eds) Handbook of functional plant ecology, 2nd edn. CRC Press, Boca Raton, pp 8–66
Kenrick P, Crane PR (1997) The origin and early evolution of plants on land. Nature 389:33–39
Knauth LP, Kennedy MJ (2009) The late Precambrian greening of the Earth. Nature 460:728–732
Kohlmeyer J, Hawksworth DL, Volkmann-Kohlmeyer B (2004) Observations on two marine and maritime “borderline” lichens: Mastodia tesellata and Collemopsidium pelvetiae. Mycol Prog 3:51–56
Kopczyński K (2006) Zapis kopalny grzybów i organizmów grzybopodobnych [Fossil record of fungi and pseudofungi]. Prz Geol 54(3):231–237
Kranner I, Cram WJ, Zorn M, Wornik S, Yoshimura I, Stabentheiner E, Pfeifhofer HW (2005) Antioxidants and photoprotection in a lichen as comparedwith its isolated symbiotic partners. Proc Natl Acad Sci 102:3141–3146
Lalley JS, Viles HA (2005) Terricolous lichens in the northern Namib Desert of Namibia: distribution and community composition. Lichenologist 37:77–91
Lang BF, Gray MW, Burger G (1999) Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet 33:351–397
Larsson SG (1978) Baltic amber – a palaeobiological study. Entomonograph vol.1. Scandinavian Science Press, Klampenborg
Lewis LA, McCourt RM (2004) Green algae and the origin of land plants. Am J Bot 91:1535–1556
Liba CM, Ferrara FIS, Mangio GP, Fantinatti-Garboggini F, Albuquerque RC, Pavan C, Ramos PL, Moreira-Filho CA, Barbosa CR (2006) Nitrogen-fixing chemo-organotrophic bacteria isolated from cyanobacteria-deprived lichens and their ability to solubilize phosphate and to release amino acids and phytohormones. J Appl Microbiol 101:1076–1086
Liu YJ, Hall BD (2004) Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc Natl Acad Sci 101:4507–4512
Lücking R, Grube M (2002) Facultative parasitism and reproductive strategies in Chroodiscus (Ascomycota, Ostropales). Stapfia 80:267–292
Lumbsch HT, Schmitt I, Palice Z, Wiklund E, Ekman S, Wedin M (2004) Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences. Mol Phylogenet Evol 31:822–832
Lutzoni F, Pagel M, Reeb V (2001) Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411:937–940
MacGinitie HD (1937) The flora of the Weaverville Beds of Trinity County, California. Carnegie Inst Wash Publ 465:83–151
Margulis L, Sagan D (1990) Origins of sex: three billion years of genetic recombination. Yale University Press, New Haven
Martin BD, Schwab E (2013) Current usage of symbiosis and associated terminology. Int J Biol 5(1):32–45
Martín MP, Coucheron DG, Johansen S (2003) Structural features and evolutionary considerations of group IB introns in SSU rDNA of the lichen fungus Teloschistes. Fungal Genet Biol 40:252–260
Matsunaga KKS, Stockey RA, Tomescu AMF (2013) Honeggeriella complexa gen. et sp. nov., a heteromerous lichen from the Lower Cretaceous of Vancouver Island (British Columbia, Canada). Am J Bot 100:450–459
McCourt RM, Delwiche CF, Karolc KG (2004) Charophyte algae and land plant origins. Trends Ecol Evol 19:661–666
McCune B, Schoch C, Root HT, Kageyama SA, Miadlikowska J (2011) Geographic, climatic, and chemical differentiation in the Hypogymnia imshaugii species complex (Lecanoromycetes, Parmeliaceae) in North America. Bryologist 114:526–544
McDonald TR, Mueller O, Dietrich FS, Lutzoni F (2013) High-throughput genome sequencing of lichenizing fungi to assess gene loss in the ammonium transporter/ammonia permease gene family. BioMed Central Genomics 14:225. doi:10.1186/1471-2164-14-225
Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lücking R, Hestmark G, Otalora MG, Rauhut A, Büdel B, Scheidegger C, Timdal E, Stenroos S, Brodo IM, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May PF, Schoch C, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98:1088–1103
Muggia L, Vancurova L, Skaloud P, Peksa O, Wedin M, Grube M (2013) The symbiotic playground of lichen thalli – a highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol Ecol 85:313–325
Myllys L, Stenroos S, Thell A, Kuusinen M (2007) High cyanobiont selectivity of epiphytic lichens in old growth foreal forest of Finland. New Phytol 173:621–629
Naeem S, Li S (1997) Biodiversity enhances ecosystem reliability. Nature 390:507–509
Nash TH (2008) Introduction. In: Nash TH (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 1–8
Niklas KJ, Kutschera U (2010) The evolution of the land plant life cycle. New Phytol 185:27–41
Okamoto N, Inouye I (2005) A secondary symbiosis in progress? Science 310:287. doi:10.1126/science.1116125
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Mycol Res 10:763–775
Peacock KA (2011) Symbiosis in ecology and evolution. In: Gabbay DM, Thagard P, Woods J (eds) Handbook of the philosophy of science: philosophy of ecology. Elsevier, Amsterdam, pp 219–250
Peréz-Ortega S, de los Ríos A, Crespo A, Sancho LG (2010) Symbiotic lifestyle and phylogenetic relationships of the bionts of Mastodia tessellata (Ascomycota, incertae sedis). Am J Bot 97:738–752
Pérez-Quintero AL, Cerón BW (2009) Lichen community structure and morphological changes in the genus Sticta (Stictaceae) associated to an altitude gradient. Acta Biol Colombiana 14:157–170
Peterson EB (2000) An overlooked fossil lichen (Lobariaceae). Lichenologist 32:298–300
Pirozynski KA, Dalphe Y (1989) Geological history of the Glomaceae with particular reference to mycorrhizal symbiosis. Symbiosis 7:1–36
Podbielkowski Z, Rejment-Grochowska I, Skirgiełło A (1979) Rośliny zarodnikowe [Spore plants]. Państwowe Wydawnictwo Naukowe, Warszawa
Poinar GO, Peterson EB, Platt JL (2000) Fossil Parmelia in New World amber. Lichenologist 32:263–269
Porembski S, Barthlott W (2000) Granitic and gneissic outcrops (inselbergs) as centers of diversity for desiccation-tolerant vascular plants. Plant Ecol 151:19–28
Prieto M, Wedin M (2013) Dating the diversification of the major lineages of Ascomycota (Fungi). PLoS One 8(6), e65576. doi:10.1371/journal.pone.0065576
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytol 156:327–349
Qiu Y-L (2008) Phylogeny and evolution of charophytic algae and land plants. J Syst Evol 46:287–306
Rambold G, Friedl T, Beck A (1998) Photobionts in lichens: possible indicators of phylogenetic relationships? Bryologist 101:392–397
Raven JA (1999) The size of cells and organisms in relation to the evolution of embryophytes. Plant Biol 1:2–12
Raven JA, Edwards D (2001) Roots: evolutionary origins and biogeochemical significance. J Expl Bot 52:381–401
Remy W, Taylor TN, Hass H, Kerp H (1994) Four hundred million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci 91:11841–11843
Renobales G, Noya R (1991) Estudio morfológico comparado de Verrucaria maura y V. amphibia en la Costa Vasca. Acta Bot Malacitana 16:149–156
Retallack GJ (1994) Were the Ediacaran fossils lichens? Paleobiology 20:523–544
Retallack GJ (1995) Ediacaran lichens – reply to Waggoner. Paleobiology 21:398–399
Retallack GJ (2014) Precambrian life on land. Palaeobotanist 63:1–15
Rikkinen J (2003) Calicioid lichens from European Tertiary amber. Mycologia 95:1032–1036
Rikkinen J, Poinar G (2000) A new species of resinicolous Chaenothecopsis (Mycocaliciaceae, Ascomycota) from 20 million year old Bitterfeld amber, with remarks on the biology of resinicolous fungi. Mycol Res 104:7–15
Rikkinen J, Poinar GO Jr (2002) Fossilized Anzia (Lecanorales, lichen-forming Ascomycota) from European Tertiary amber. Mycol Res 106:984–990
Rikkinen J, Poinar GO Jr (2008) A new species of Phyllopsora (Lecanorales, lichen-forming Ascomycota) from Dominican amber, with remarks on the fossil history of lichens. J Expl Bot 59:1007–1011
Romeike J, Friedl T, Helms G, Ott S (2002) Genetic diversity of algal and fungal partners in four species of Umbilicaria (Lichenized Ascomycetes) along a transect of the Antarctic peninsula. Mol Biol Evol 19:1209–1217
Rundel PW (1978) Ecological relationships of desert fog zone lichens. Bryologist 81:277–293
Schlensog M, Schroeter B (2000) Poikilohydry in Antarctic cryptogams and its influence on photosynthetic performance in mesic and xeric habitats. In: Davison W, Howard-Williams C, Broady P (eds) Antarctic ecosystems: models for a wider ecological understanding. Caxton, Christchurch, pp 175–182
Schoch CL, Crous PW, Groenewald JZ, Boehm EW, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EB, Kohlmeyer J, Kruys A, Li YM, Lücking R, Lumbsch HT, Marvanova L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJ, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Plata ER, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JH, Yonezawa H, Zhang Y, Spatafora JW (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Schöller H (1997) Systematik der Flechten. In: Schöller H (ed) Flechten. Geschichte, Biologie, Systematik, Ökologie, Naturschutz und kulturelle Bedeutung. Senckenberg Naturf Gesellschaft 27:68–82
Schwab AJ (1986) Rostfarbene Arten der Sammelgattung Lecidea (Lecanorales). Revision der Arten Mittel- und Nordeuropas. Mitt Bot Staatssamml München 22:221–476
Seaward MRD (1988) Contribution of lichens to ecosystems. In: Galun M (ed) CRC handbook of lichenology, vol 2. CRC Press, Boca Raton, pp 107–129
Selosse M-A, Le Tacon F (1998) The land flora: a phototroph-fungus partnership? Trends Ecol Evol 13:15–20
Simon L, Bousquet J, Lévesque RC, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363:67–69
Ślusarczyk E (1996) Ewolucja strategii życiowych Glomales [Evolution of life strategies in Glomales]. Wiadomości Botaniczne 40:29–35
Smith AL (1921) Lichens. Cambridge University Press, Cambridge
Speidel M (2000) The parasitic host: symbiosis contra neo-Darwinism. Pli 9:119–138
Steudel B, Hector A, Friedl T, Löfke C, Lorenz M, Wesche M, Kessler M, Gessner M (2012) Biodiversity effects on ecosystem functioning change along environmental stress gradients. Ecol Lett 15:1397–1405
Stubblefield SP, Taylor TN, Trappe JM (1987) Fossil mycorrhizae: a case for symbiosis. Science 237:59–60
Summerfield TC, Eaton-Rye JJ (2006) Pseudocyphellaria crocata, P. neglecta and P. perpetua from the Northern and Southern Hemispheres are a phylogenetic species and share cyanobionts. New Phytol 170:597–607
Syvanen M (1986) Cross-species gene transfer: a major factor in evolution? Trends Genet 107:685–696
Taylor TN, Hass H, Remy W, Kerp H (1995) The oldest fossil lichen. Nature 378:244
Tehler A (1996) Systematics, phylogeny and classification. In: Nash TH (ed) Lichen biology. Cambridge University Press, Cambridge, pp 217–239
Tehler A, Wedin M (2008) Systematics of lichenized fungi. In: Nash TH (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 336–352
Tester M, Smith SE, Smith FA (1987) The phenomenon of “nonmycorrhizal” plants. Can J Bot 65:419–431
Thüs H, Aptroot A, Seaward MRD (2014) Freshwater lichens. In: Jones EBG, Hyde KD, Pang K-L (eds) Freshwater fungi and fungal-like organisms. De Gruyter, Berlin, pp 333–358
Toole G, Toole S, Toole SM (2004) Essential A2 biology for OCR. Nelson Thomas, Cheltenham
Tunjić M, Korać P (2013) Vertical and horizontal gene transfer in lichens. Period Biol 115:321–329
Turnau K, Jurkiewicz A, Grzybowska B (2002) Rola mikoryzy w bioremediacji terenów zanieczyszczonych [The role of mycorrhiza in bioremediation of polluted sites]. Kosmos 2:185–194
Vinebrooke RD, Cottingham KL, Norberg J, Scheffer M, Dodson SI, Maberly SC, Sommer U (2004) Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104:451–457
Waggoner BM (1995) Ediacaran lichens: a critique. Paleobiology 21:393–397
Walter H, Kreeb H (1970) Die Hydration und Hydratur des Protoplasmas der Pflanzen und ihre öko-physiologische Bedeutung. Springer, Vienna
Wedin M, Döring H, Gilestam G (2004) Saprotrophy and lichenization as options for the same fungal species on different substrata: environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytol 164:459–465
Wernegreen JJ (2004) Endosymbiosis: lessons in conflict resolution. PLoS Biol 2, e68. doi:10.1371/journal.pbio.0020068
Williams TA, Foster PG, Nye TMW, Cox CJ, Embley TM (2012) A congruent phylogenomic signal places eukaryotes within the Archaea. Proc R Soc B 279:4870–4879
Wirth V (1972) Die Silikatflechten-Gemeinschaften im ausseralpinen Zentraleuropa. Dissertationes Botanicae, Lehre 17:213–240
Witzany G (2010) Biocommunication and natural genome editing. Springer, Dordrecht
Yuan X, Xiao S, Taylor TN (2005) Lichen-like symbiosis 600 million years ago. Science 308:1017–1020
Acknowledgments
I am most grateful to Prof. David Richardson (Halifax, Canada) and Prof. Mark Seaward (Bradford, England) for their most helpful criticism and linguistic correction of draft versions of this paper and to Robert Janczar and Dr Michał Lipnicki for extensive help and linguistic advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lipnicki, L.I. The role of symbiosis in the transition of some eukaryotes from aquatic to terrestrial environments. Symbiosis 65, 39–53 (2015). https://doi.org/10.1007/s13199-015-0321-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-015-0321-7