Abstract
Introduction
The objective of this study was to investigate the epidemiology of dietary supplement exposures in the USA.
Methods
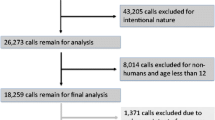
A retrospective analysis was conducted of out-of-hospital dietary supplement exposures reported to the National Poison Data System from 2000 through 2012.
Results
There were 274,998 dietary supplement exposures from 2000 through 2012. The annual rate of dietary supplement exposures per 100,000 population increased by 46.1% during 2000–2002, decreased 8.8% during 2002–2005, and then increased again by 49.3% from 2005 to 2012. These trends were influenced by the decrease in ma huang exposures starting in 2002. Miscellaneous dietary supplements accounted for 43.9% of all exposures, followed by botanicals (31.9%), hormonal products (15.1%), and other supplements (5.1%). The majority of dietary supplement exposures (70.0%) occurred among children younger than 6 years old and were acute (94.0%) and unintentional (82.9%). Serious medical outcomes accounted for 4.5% of exposures and most (95.0%) occurred among individuals 6 years and older. Ma huang products, yohimbe, and energy products were the categories associated with the greatest toxicity.
Conclusions
There was an overall increase in the rate of dietary supplement exposures from 2000 through 2012. Although the majority of these exposures did not require treatment at a health care facility or result in serious medical outcomes, exposures to yohimbe and energy products were associated with considerable toxicity. Our results demonstrate the success of the FDA ban on ma huang products and the need for FDA regulation of yohimbe and energy products in the USA.



Similar content being viewed by others
Abbreviations
- AAPCC:
-
American Association of Poison Control Centers
- AMA:
-
Against medical advice
- CCU:
-
Critical care unit
- FDA:
-
Food and Drug Administration
- HCF:
-
Health care facility
- NPDS:
-
National Poison Data System
- PCC:
-
Poison control center
- TESS:
-
Toxic Exposure Surveillance System
- US:
-
United States
References
U.S. Food and Drug Administration. Dietary Supplement Health and Education Act of 1994. https://ods.od.nih.gov/About/DSHEA_Wording.aspx. Accessed December 29, 2016.
Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988-1994). NCHS Data Brief 2011(61):1–8.
Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316(14):1464–74.
Dangerous supplements: Still at large. Consumer Reports. April 24, 2004. http://consumersunion.org/pub/0504%20DietarySup.pdf. Accessed January 11, 2017.
Sadovsky R, Collins N, Tighe AP, Brunton SA, Safeer R. Patient use of dietary supplements: a clinician's perspective. Curr Med Res Opin. 2008;24(4):1209–16.
U.S. Food and Drug Administration. Guidance for clinical investigators, sponsors, and IRBs: Investigational new drug applications (INDs)--Determining whether human research studies can be conducted without an IND. 2013. http://www.fda.gov/downloads/drugs/guidances/ucm229175.pdf. Accessed December 29, 2016.
Zelig R, Rigassio RD. Understanding the properties of common dietary supplements: clinical implications for healthcare practitioners. Nutr Clin Pract. 2012;27(6):767–76.
Gryzlak BM, Wallace RB, Zimmerman MB, Nisly NL. National surveillance of herbal dietary supplement exposures: the poison control center experience. Pharmacoepidemiol Drug Saf. 2007;16(9):947–57.
Haller C, Kearney T, Bent S, Ko R, Benowitz N, Olson K. Dietary supplement adverse events: report of a one-year poison center surveillance project. J Med Toxicol. 2008;4(2):84–92.
Palmer ME, Haller C, McKinney PE, Klein-Schwartz W, Tschirgi A, Smolinske SC, et al. Adverse events associated with dietary supplements: an observational study. Lancet. 2003;361(9352):101–6.
Seifert SM, Seifert SA, Schaechter JL, Bronstein AC, Benson BE, Hershorin ER, et al. An analysis of energy-drink toxicity in the National Poison Data System. Clin Toxicol (Phila). 2013;51(7):566–74.
Woolf AD, Watson WA, Smolinske S, Litovitz T. The severity of toxic reactions to ephedra: comparisons to other botanical products and national trends from 1993-2002. Clin Toxicol (Phila). 2005;43(5):347–55.
Geller AI, Shehab N, Weidle NJ, Lovegrove MC, Wolpert BJ, Timbo BB, et al. Emergency department visits for adverse events related to dietary supplements. N Engl J Med. 2015;373(16):1531–40.
Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila). 2013;51(10):949–1229.
U.S. Census Bureau. Intercensal estimates of the resident population by single year of age, sex, race, and Hispanic origin for the United States: April 1, 2000-July 1, 2010 2012. http://www.census.gov/data/tables/time-series/demo/popest/intercensal-2000-2010-national.html. Accessed January 3, 2017.
Kinariwala N. Tighter regulation needed for dietary supplements in USA. Lancet. 2003;361(9368):1566.
Polivka BJ, Elliott MB, Wolowich WR. Comparison of poison exposure data: NHIS and TESS data. J Toxicol Clin Toxicol. 2002;40(7):839–45.
Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72(2 Suppl):624S–36S.
WebMD. Find a vitamin or supplement: Yohimbe. http://www.webmd.com/vitamins-supplements/ingredientmono-759-yohimbe.aspx?activeingredientid=759&. Accessed December 29, 2016.
U.S. Food and Drug Administration. FDA issues regulation prohibiting sale of dietary supplements containing ephedrine alkaloids and reiterates its advice that consumers stop using these products. 2004. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2004/ucm108242.htm. Accessed December 29, 2016.
Wolk BJ, Ganetsky M, Babu KM. Toxicity of energy drinks. Curr Opin Pediatr. 2012;24(2):243–51.
Sepkowitz KA. Energy drinks and caffeine-related adverse effects. JAMA. 2013;309(3):243–4.
Danno K, Colas A, Masson JL, Bordet MF. Homeopathic treatment of migraine in children: results of a prospective, multicenter, observational study. J Altern Complement Med. 2013;19(2):119–23.
Pellow J, Solomon EM, Barnard CN. Complementary and alternative medical therapies for children with attention-deficit/hyperactivity disorder (ADHD). Altern Med Rev. 2011;16(4):323–37.
Torres-Llenza V, Bhogal S, Davis M, Ducharme F. Use of complementary and alternative medicine in children with asthma. Can Respir J. 2010;17(4):183–7.
Pitetti R, Singh S, Hornyak D, Garcia SE, Herr S. Complementary and alternative medicine use in children. Pediatr Emerg Care. 2001;17(3):165–9.
Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25(5):246–54.
Beer AM, Burlaka I, Buskin S, Kamenov B, Pettenazzo A, Popova D, Riveros Huckstadt MP, Sakalinskas V, Oberbaum M. Usage and Attitudes Towards Natural Remedies and Homeopathy in General Pediatrics: A Cross-Country Overview. Glob Pediatr Health. 2016;3:2333794X15625409.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no conflicts of interest or financial disclosures relevant to this study to disclose.
Sources of Funding
None.
Financial Disclosure
The authors have no financial disclosures relevant to this study.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Rao, N., Spiller, H.A., Hodges, N.L. et al. An Increase in Dietary Supplement Exposures Reported to US Poison Control Centers. J. Med. Toxicol. 13, 227–237 (2017). https://doi.org/10.1007/s13181-017-0623-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-017-0623-7