Abstract
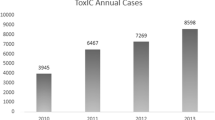
The American College of Medical Toxicology established the Toxicology Investigators Consortium (ToxIC) Case Registry in 2010. The Registry contains all medical toxicology consultations performed at participating sites. The Registry has continued to grow since its inception, and as of December 31, 2015, contains 43,099 cases. This is the sixth annual report of the ToxIC Registry, summarizing the additional 8115 cases entered in 2015. Cases were identified by a query of the Registry for all cases entered between January 1 and December 31, 2015. Specific data reviewed for analysis included demographics (age, race, gender), source of consultation, reason for consultation, agents and agent classes involved in exposures, signs, symptoms, clinical findings, fatalities, and treatment. By the end of 2015, there were 50 active sites, consisting of 101 separate health-care facilities; 51.2 % of cases involved females. Adults between the ages of 19 and 65 made up the majority (64.2 %) of Registry cases. Caucasian race was the most commonly reported (55.6 %); 9.6 % of cases were identified as Hispanic ethnicity. Inpatient and emergency department referrals were by far the most common referral sources (92.9 %). Intentional pharmaceutical exposures remained the most frequent reason for consultation, making up 52.3 % of cases. Of these intentional pharmaceutical exposures, 69 % represented an attempt at self-harm, and 85.6 % of these were a suicide attempt. Nonopioid analgesics, sedative-hypnotics, and antidepressant agents were the most commonly reported agent classes in 2015. Almost one-third of Registry cases involved a diagnosed toxidrome (32.8 %), with a sedative-hypnotic toxidrome being the most frequently described. Significant vital sign abnormalities were recorded in 25.3 % of cases. There were 98 fatalities reported in the Registry (1.2 %). Adverse drug reactions were reported in 4.3 % of cases. Toxicological treatment was given in 65.3 % of cases, with 33.0 % receiving specific antidotal therapy. Exposure characteristics and trends overall were similar to prior years. While treatment interventions were required in the majority of cases, fatalities were rare.

Similar content being viewed by others
References
Wax PM, Kleinschmidt KC, Brent J. ACMT ToxIC Case Registry Investigators. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–65.
Finkelstein Y, Goel G, Hutson JR, Armstrong J, Baum CR, et al. Drug misuse in adolescents presenting to the emergency department. Pediatr Emerg Care. 2015. doi:10.1097/PEC.0000000000000571.
Froberg BA, Levine M, Beuhler MC, Judge BS, et al. Acute methylenedioxypyrovalerone toxicity. J Med Toxicol. 2015;11(2):185–94.
Rhyee SH, Farrugia L, Campleman SL, et al. The Toxicology Investigators Consortium Case Registry—the 2014 experience. J Med Toxicol. 2015;11(4):388–409.
Smith SW, Farmer BM. Toxicology in the service of patient and medication safety: a selected glance at past and present innovations. J Med Toxicol. 2015;11(2):245–52.
Watkins JW, Schwarz ES, Arroyo-Plasencia AM, Mullins ME. The use of physostigmine by toxicologists in anticholinergic toxicity. J Med Toxicol. 2015;11(2):179–84.
Zelner I, Matlow J, Hutson JR, Wax P, et al. Acute poisoning during pregnancy: observations from the Toxicology Investigators Consortium. J Med Toxicol. 2015;11(3):301–8.
Rhyee SH, Farrugia L, Wiegand T, et al. The Toxicology Investigators Consortium Case Registry—the 2013 experience. J Med Toxicol. 2014;10(4):342–59.
Wiegand TJ, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol. 2012;8(4):360–77.
Wiegand T, Wax P, Smith E, et al. The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol. 2013;9(4):380–404.
Brent J, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2010 experience. J Med Toxicol. 2011;7(4):266–76.
Bronstein AC, Spyker DA, Cantilena LR, et al. 2010 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol. 2011;49(10):910–41.
Bronstein AC, Spyker DA, Cantilena LR, et al. 2011 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol. 2012;50(10):911–1164.
Mowry JB, Spyker DA, Cantilena LR, et al. 2012 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229.
Mowry JB, Spyker DA, Cantilena LR, et al. 2013 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st annual report. Clin Toxicol. 2014;52(10):1032–283.
Mowry JB, Spyker DA, Brooks DE, et al. 2014 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol. 2015;53(10):962–1147.
Acknowledgments
The authors express their sincere gratitude to the staff at the American College of Medical Toxicology for supporting the ToxIC Registry project. We very much appreciate the contribution to the Registry from each of the ToxIC sites. The following is a list of the principle coordinators from each site contributing cases in 2015.
Toxicology Investigators Consortium
Albuquerque, NM
Steven Seifert, MD
Atlanta, GA
Adam Pomerleau, MD
Ziad Kazzi, MD
Boston, MA (Beth Israel Deaconess Medical Center)
Michael Ganetsky, MD
Boston, MA (Children’s Hospital of Boston)
Michele Burns, MD
Charlotte, NC
Michael Beuhler, MD
Charlottesville, VA
Joshua D. King, MD
Chicago, IL
Steven Aks, DO
Dallas, TX
Jakub Furmaga, MD
Paul Wax, MD
Denver, CO
Jeffrey Brent, MD
Christopher Hoyte, MD
Evanston, IL
Jerrold Leikin, MD
Fresno, CA
Rais Vohra, MD
Grand Rapids, MI
Bryan Judge, MD
Brad Riley, MD
Greenville, SC
William J Meggs, MD
Haifa, Israel
Didi Bentur, MD
Harrisburg, PA
Phil Moore, MD
Hartford, CT
Lynn Farrugia, MD
Houston, TX
Spencer Greene, MD
Indianapolis, IN
Daniel Rusyniak, MD
Kansas City, MO
Jennifer Lowry, MD
Adam Algren, MD
Loma Linda, CA
Brian Wolk, MD
Los Angeles, CA
Michael Levine, MD
Manhasset, NY
Josh Nogar, MD
Milwaukee, WI
Mark Kostic, MD
David Gummin, MD
Morristown, NJ
Diane Calello, MD
New Brunswick, NJ
Ann-Jeannette Geib, MD
New York, NY (Mount Sinai Hospital)
Stephanie Hernandez, MD
New York, NY (NYU Langone Medical Center)
Silas Smith, MD
New York, NY (Bellevue Medical Center)
Lewis Nelson, MD
Newark, NJ
Steven Marcus, MD
Omaha, NE
Ronald Kirschner, MD
Philadelphia (Einstein Medical Center)
Adam Rowden, MD
Philadelphia, PA (Hahnemann University Hospital)
David Vearrier, MD
Rita McKeever, MD
Phoenix, AZ
Michelle Ruha, MD
Pittsburgh, PA
Anthony Pizon, MD
Portland, OR
Robert Hendrickson, MD
Nate McKeown, MD
Richmond, VA
Brandon Wills, DO
Kirk Cumpston, DO
Riyadh, Saudi Arabia
Mohammed Alhelail, MD
Rochester, NY
Timothy Wiegand, MD
Salt Lake City, UT
E. Martin Caravati, MD
San Antonio, TX
Daniel Sessions, MD
Joseph Maddry, MD
San Diego, CA
Alicia Minns, MD
San Francisco, CA
Derrick Lung MD
Craig Smollin MD
St. Louis, MO
Thomas Kibby, MD
Evan Schwarz MD
St. Paul, MN
Samuel Stellpflug, MD
Kristin Engebretsen, MD
Staten Island, NY
Nima Majlesi, MD
Syracuse, NY
Ross Sullivan, MD
Tampa, FL
Tamas Peredy, MD
Toronto, Canada
Yaron Finkelstiein, MD
Worcester, MA
Jennifer Carey, MD
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of Interest
None.
Funding Sources
This study received funding from the NIH National Institute of Neurological Disorders and Stroke 3U01NS083452-01 (Bird, SB), NIH National Institute on Drug Abuse 1R56DA38366 (Boyer, EW/ Carlson, RG) and 1R01DA037317-01 (Manini, A), and BTG International Inc.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 28 kb)
ESM 2
(DOCX 13 kb)
ESM 3
(DOCX 29 kb)
ESM 4
(DOCX 12 kb)
ESM 5
(DOCX 36 kb)
ESM 6
(DOCX 12 kb)
ESM 7
(DOCX 37 kb)
ESM 8
(DOCX 28 kb)
ESM 9
(DOCX 28 kb)
ESM 10
(DOCX 35 kb)
ESM 11
(DOCX 50 kb)
ESM 12
(DOCX 43 kb)
ESM 13
(DOCX 43 kb)
ESM 14
(DOCX 37 kb)
ESM 15
(DOCX 42 kb)
ESM 16
(DOCX 29 kb)
ESM 17
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Farrugia, L.A., Rhyee, S.H., Campleman, S.L. et al. The Toxicology Investigators Consortium Case Registry—the 2015 Experience. J. Med. Toxicol. 12, 224–247 (2016). https://doi.org/10.1007/s13181-016-0580-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-016-0580-6