Abstract
Age-related macular degeneration (AMD) is an ophthalmologic disease which usually affects older adults and represents the leading cause of legal blindness in Europe and the United States of America. The pathogenesis of AMD is complex and, nowadays, the treatments are targeting more the late form of the disease. Age and genetic make-up are the most important risk factors identified to date. There are undoubtedly environmental and other risk factors involved and the adverse effect of smoking is well established. New treatments for AMD have emerged with improved prognostic outcome. This remarkable advances in our understanding of the genetic and biological foundations of this disease were derived from a recent convergence of scientific and clinical data. In the near future we will have several therapeutic options for treatment of AMD at different stages and therefore personalising more and more the treatment.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is an ophthalmologic disease which usually affects older adults and results in a loss of vision in the center of the visual field, because of damage to the central retina, called the macula. It occurs in a “dry” (non-neovascular) and a “wet” (neovascular) form, the wet form being more sight-threatening. AMD can make it difficult or impossible to read or recognise faces, although enough peripheral vision remains to allow other activities of daily life. AMD starts with characteristic yellow deposits (drusen) in the macula between the retinal pigment epithelium and the underlying choroid. At this early stage of the disease experts are speaking of age-related maculopathy rather then AMD. If the drusen are becoming more numerous, geographic atrophy or marked pigmentary changes are occurring the term AMD is applied. From good vision in earlier stages the visual acuity will drop in later stages with preserved peripheral vision.
AMD is the leading cause of legal blindness in Europe [1, 2] and the United States of America. Worldwide about 30 million people are affected by the disease. Especially, the prevalence and incidence of AMD is increasing in individuals older than 50 years old. According to the demographic evolution of the population the amount of people with AMD will grow even more. The pathogenesis of AMD is complex and, nowadays, the treatments are targeting more the late form of the disease. Age and genetic make-up are the most important risk factors identified to date. There are undoubtedly environmental and other risk factors involved and the adverse effect of smoking is well established.
Predictive diagnostics and risk factors for AMD
The gold standart for AMD diagnostics is primarily a fundus examination of the macula (central area of the retina). Additionally, fluorescein angiography (FLA) and optical coherence tomography (OCT) are used to assess the disease. FLA is a technique for examining the circulation of the retina using the dye tracing method. It involves injection of sodium fluorescein into the systemic circulation, and then an angiogram is obtained by photographing the fluorescence emitted after illumination of the retina. OCT is an optical signal acquisition and processing method using the interferometric technique and producing scans of the posterior part of the eye. These three investigations are helping the ophthalmologist to differentiate between the two main forms of AMD, dry (non-neovascular) and wet (neovascular). Follow-up controls will then characterise the disease course, help to predict the further evolution and imply an adjusted management of the patient.
Nowadays the etiology of AMD is believed to be an interaction of environmental impacts and genetic factors. Newest investigations show that AMD is probably caused in two-third of the cases by genetic predisposition.
Ocular and systemic risk factors
Iris color, hair color, and skin sun sensitivity are not associated with the development of late forms of AMD [3]. Other ocular factors like refraction and cataract seem not to be a risk factor for AMD. Only a small number of published studies investigated the development or progression of AMD following cataract surgery. The results were inconsistent, nevertheless a promoting influence of cataract surgery on the progression of early age-related macular degeneration can be assumed [4].
Some studies showed a correlation between cardiovascular risk factors and progression of AMD, but the results were not consistent. In histological examinations accumulation of lipids with age appears to be greater in the central fundus than in the peripheral region, indicating that lesions in AMD and Bruch’s membrane lipid deposits with age share a common spatial distribution, but there is no clear correlation between blood cholesterol and higher risk for AMD [5]. Regarding the association among hyperglycaemia, diabetes status, and age-related maculopathy large studies proved that diabetes is not related to early age-related maculopathy or dry forms of AMD [6].
External risk factors
AMD involves a progressive impairment of the outer layers, especially photoreceptors, in the centre of the retina. Experimental studies have demonstrated that bright light preferentially damages precisely the region that degenerates in AMD. Although the physiological pigments melanin, lutein and zeaxanthin are acting as photoprotectant in the macula, solar radiation is responsible for some of the deteriorative changes that lead to AMD. In the human retina, documented lesions from solar radiation range from the acute effects of sun-gazing to injuries resulting from prolonged periods of exposure in brightly illuminated environments. The damage occurs in the same region that degenerates in AMD [7]. Nevertheless, it is difficult to prove the effect of light promoted AMD (Figs. 1, 2 and 3).
Same eye and patient as in Fig. 1. Optical coherence tomography (OCT) showing the atrophy of the retina and the subretinal scar tissue
Same eye and patient as in Fig. 1. Fluorescent angiography (FLA) late phase reveals the large area of the involved tissue in the macula
In a pooled three-continent study there is a growing body of evidence indicating that smoking is related to an increased risk of incident AMD and is the leading external risk factor for AMD. Smoking is associated with a two-fold increased risk of developing AMD. Current smokers were at higher risk of incident AMD than both past smokers and those who never smoked [8, 9]. Some studies could even demonstrate a strong association between the risk of advanced forms of AMD, both dry and wet, and pack years of cigarette smoking. There seem to be even an increased risk for AMD in non-smokers exposed to passive smoking. Stopping smoking appears to reduce the risk of developing AMD [10, 11]. Smoking reduces the concentration of antioxidant in the blood and probably also the function of antioxidant enzymes in the macula. Additionally, Factor H being an important element of the pathogenesis of AMD shows an age-dependent increase of the plasma level and a decrease in smokers [12]. However, the exact mechanism how smoking is influencing AMD needs still to be elucidated.
Lipofuscin and vascular endothelial growth factor (VEGF)
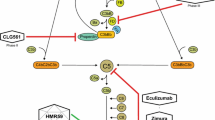
In the pathogenesis of AMD many mechanism are discussed. Regarding the two main forms, dry and wet AMD, the formation of lipofuscin plays an important role for the non-neovascular form and pro- and antiangiogenic factors more for the neovascular form. Lipofuscin is considered one of the ageing pigments, found in the liver, kidney, heart muscle, adrenals, nerve cells and pigment epithelium of the eye. It is specifically arranged around the nucleus, and is a type of lipochrome. Lipofuscin accumulation in the retinal pigment epithelium is associated with various blinding retinal diseases, including AMD. The major lipofuscin fluorophor A2-E is thought to play the most important pathogenetic role. It has been shown that A2-E severely impairs lysosomal function of RPE cells as a potent inhibitor of the ATP-driven proton pump located in the lysosomal membrane. Such inhibition of proton transport to the lysosomal lumen results in an increase of the lysosomal pH with subsequent inhibition of lysosomal hydrolases. An essential task of the lysosomal apparatus of retinal pigment epithelium cells for normal photoreceptor function is phagocytosis and degradation of membranous discs shed from photoreceptor outer segments and of biomolecules from autophagy. When lysosomes of cultured retinal pigment epithelium cells were experimentally loaded with A2-E, there was an intracellular accumulation of exogenously added photoreceptor outer segments. Moreover, the autophagic sequestration of cytoplasmic material was also markedly reduced after A2-E loading. These data support the hypothesis that A2-E-induced lysosomal dysfunction contributes to the pathogenesis of AMD [13].
Vascular endothelial growth factor (VEGF) is a chemical signal produced by cells that stimulates the growth of new blood vessels. It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. VEGF’s normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and to bypass blocked vessels. When VEGF is overexpressed, it contributes to the wet form of AMD. VEGF blocking with VEGF-inhibitors like ranibizumab or bevacizumab has revolutionised the treatment of neovascular AMD since 2006 [14]. The treatment is stabilising the disease and visual acuity in many cases, although repetitive intravitreal applications are necessary in most of the cases.
Genetics
Age and genetic make-up are the most important risk factors for AMD identified to date. Over the next decade, the different genes that are involved in the development of age-related macular degeneration will be identified [15]. Many studies suggested a genetic implication for AMD. For example the prevalence of age-related maculopathy among first-degree relatives of subjects with age-related maculopathy, particularly with exudative disease, is greater then among first-degree relatives of subjects without this disease [16]. Another interesting point is that genetic susceptibility may play an important role in determining the onset of disease and therefore a better understanding of the genetic factors in AMD would contribute to understanding the pathogenesis [17]. If those at risk could be identified it may be possible to modify lifestyle or develop novel therapies in the presymptomatic stage to prevent disease or decrease its severity.
Several hereditary retinal dystrophies show similarities to AMD and these genes are potential candidate susceptibility genes. Particular interest has focused on the ABCR gene which is responsible for autosomal recessive Stargardt macular dystrophy. It has been claimed that heterozygotes for ABCR mutations are predisposed to AMD but the data are conflicting. Studies of the genes responsible for autosomal dominant Sorsby fundus dystrophy, Doyne honeycomb retinal dystrophy, and Best disease have given negative results. In one large AMD family, linkage has been reported to markers in 1q25-q31. Recent data suggest that the ApoE epsilon4 allele may be associated with reduced risk of AMD [18]. Linkage and association studies have identified other chromosomal regions that are likely to contain susceptibility loci with strongest evidence found on chromosome 1q31 and 10q26. Variants in the complement factor H (CFH) gene have been shown by several independent studies to be associated with an increased risk for AMD in Caucasian populations. Factor H is a member of the regulators of complement activation family functioning as a complement control protein. Its main job is to regulate the alternative pathway of the complement system, ensuring that the complement system is directed towards pathogens and does not damage host tissue. The implication of complement factor H shows that the innate immune system may play a significant role in AMD pathogenesis. The LOC387715/HTRA1 locus within 10q26 has been identified as a second major locus contributing to AMD pathogenesis [19].
It can be argued that age-related macular degeneration is one of the best characterised complex trait diseases. Extensive information related to genetic and environmental risk factors exists, and a number of different biological pathways are strongly implicated in its aetiology. Along with recent improvements in high throughput and relatively inexpensive genetic technologies, we are now in a position to consider developing a presymptomatic, personalised approach towards the assessment, management and treatment of this disease. We explore the applicability and challenges of this approach if it is to become commonplace for guiding treatment decisions for individuals with pre-existing disease or for those at high risk of developing it.
Prevention and prophylaxis
Vitamines
Oxidative stress, which refers to cellular damage caused by reactive oxygen intermediates (ROI), has been implicated in many disease processes, especially age-related disorders. ROIs include free radicals, hydrogen peroxide, and singlet oxygen, and they are often the byproducts of oxygen metabolism. The retina is particularly susceptible to oxidative stress because of its high consumption of oxygen, its high proportion of polyunsaturated fatty acids, and its exposure to visible light. In vitro studies have consistently shown that photochemical retinal injury is attributable to oxidative stress and that the antioxidant vitamins A, C, and E protect against this type of injury. Furthermore, there is strong evidence suggesting that lipofuscin is derived, at least in part, from oxidatively damaged photoreceptor outer segments and that it is itself a photoreactive substance. However, the relationships between dietary and serum levels of the antioxidant vitamins and age-related macular disease are less clear, although a protective effect of high plasma concentrations of alpha-tocopherol has been convincingly demonstrated [20]. Macular pigment as mentioned before is also believed to limit retinal oxidative damage by absorbing incoming blue light and/or quenching ROIs. Many putative risk-factors for AMD have been linked to a lack of macular pigment, including female gender, lens density, tobacco use, light iris color, and reduced visual sensitivity. Moreover, the Eye Disease Case-Control Study found that high plasma levels of lutein and zeaxanthin were associated with reduced risk of neovascular AMD. The concept that AMD can be attributed to cumulative oxidative stress is enticing, but remains unproven. With a view to reducing oxidative damage, the effect of nutritional antioxidant supplements on the onset and natural course of age-related macular disease is currently being evaluated [21]. Lutein and zeaxanthin are concentrated at the macula, where they are collectively known as macular pigment, and where they are believed to play a major role in protecting retinal tissues against oxidative stress. Whilst the exact pathogenesis of AMD remains unknown, the disruption of cellular processes by oxidative stress may play an important role. Manipulation of dietary intake of lutein and zeaxanthin has been shown to change macular pigment concentration, thereby raising hopes that dietary supplementation with these carotenoids might prevent, delay, or modify the course of AMD [22]. Lipids are known to accumulate in Bruch’s membrane, an acellular layer with no known intrinsic mechanisms to combat lipid peroxidation. In related studies, lipid peroxides have been shown to induce neovascularisation by inducing expression of a cascade of angiogenic cytokines. Lipid peroxides are biological molecules that have the potential to incite new vessel growth. The increase in amount of peroxidised lipids with age, combined with their vasogenic potential, suggests that peroxidised lipids may play a role in the aetiology of age-related macular degeneration, particularly choroidal neovascularisation [23].
Zinc
Zinc is an essential mineral of very important biologic and public health importance. Zinc deficiency affects about two billion people in the developing world and is associated with many diseases. Zinc has long been recognised as an essential constituent of various tissues. Many clinical conditions and dietary factors reduce the absorption or the biological availability of zinc, and lead to zinc deficiency which produces structural and functional alterations in many organ systems. The highest concentration of this trace element in the human body is measured in the eye, particularly in the pigment-containing components. The deficiency of zinc has a dramatic effect on ocular development especially when it occurs during early prenatal period [24, 25]. Zinc is required for the structure and activity of many ocular metalloenzymes and plays a role in the metabolic function of several important enzymes in the chorioretinal complex. Although the exact mechanism of its molecular and cellular functions are largely unknown the essentiality of this element in the component of the eye, including the retina, choroid, cornea and lens, is well established; it is also well known that zinc deficiency causes functional impairments in various parts of the eye. Zinc related toxicities have been shown in human and animal eyes [26]. A controlled oral intervention study showed a positive, if limited, treatment effect in AMD. Because of the possible toxic effects and complications of oral zinc administration, widespread use of zinc in macular degeneration is not now warranted.
In 2001 the “Age-Related Eye Disease Study” (AREDS), one of the biggest studies ever performed in the ophthalmologic field regarding vitamins and micronutrients has been published [27]. 3640 patients have been involved and followed for more than 6 years. The goal of the study was to demonstrate the effect of antioxidative vitamins in high-doses (vitamine C, Betacarotin, Vitamin E) and the micronutrient zinc (combined with copper) on progression of AMD. According to the clinical fundus picture patients have been put into four categories, the first being beginning and the last advanced AMD. The patients have been randomised into four groups with group 1 only antioxidative vitamins, group 2 only zinc (and cupper), group 3 combination of vitamins and zinc and group 4 placebo. Both, zinc and antioxidants plus zinc significantly reduced the odds of developing advanced AMD in the two advanced AMD categories and the only statistically significant reduction in rates of at least moderate visual acuity loss occurred in persons assigned to receive antioxidants plus zinc [27]. Therefore the recommendation was that persons with intermediate or advanced form of AMD in one eye should consider taking the AREDS-type supplements. Further evaluation of nutritional factors, specifically, lutein/zeaxanthin and omega-3 fatty acids will be tested in another large multicenter controlled, randomised trial—the Age-Related Eye Disease Study 2 (AREDS2) [28]. In an animal model increasing omega-3-PUFA tissue levels by dietary or genetic means decreased the avascular area of the retina by increasing vessel regrowth after injury, thereby reducing the hypoxic stimulus for neovascularisation. The bioactive omega-3-PUFA-derived mediators neuroprotectinD1, resolvinD1 and resolvinE1 also potently protected against neovascularisation. The protective effect of omega-3-PUFAs and their bioactive metabolites was mediated, in part, through suppression of tumour necrosis factor-alpha. These findings indicate that increasing the sources of omega-3-PUFA or their bioactive products reduces pathological angiogenesis which could influence the course of AMD [29]. Omega-3 (n-3) Long-chain polyunsaturated fatty acids (LCPUFAs) affect processes implicated in vascular and neural retinal pathogenesis and thus may influence the risk of developing age-related macular degeneration (AMD). The 12-y incidence of dry and wet AMD in participants at moderate-to-high risk of these outcomes was lowest for those reporting the highest consumption of omega-3 LCPUFAs [30].
Antiinflammatory, antioxidative and other substances
Tyrosinkinase inhibitors
Tyrosinkinase inhibitors are interfering with the signal transduction of growth hormons. The tyrosinkinase inhibitor genistein found in a number of plants, with lupin, fava beans, soybeans, kudzu, and psoralea being the primary food source acts as antioxidant, similar to many other isoflavones, counteracting damaging effects of free radicals in tissues. The isoflavonoid genistein is a natural tyrosinkinase inhibitor and is very potent in inhibition of endothelial cell proliferation and in vitro angiogenesis. Moreover, genistein inhibits the proliferation of various tumour cells. Genistein excretion in urine of subjects consuming a plant-based diet is in the micromolar range, which is 30-fold higher than that of subjects consuming a traditional Western diet. Thus genistein with tyrosinkinase inhibiting and antioxidant properties may have important applications in the treatment of solid tumours and angiogenic diseases [31, 32].
Statins
The statins or HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase) inhibitors are a class of drug used to lower plasma cholesterol level. Inhibition of HMG-CoA reductase in the liver results in decreased cholesterol synthesis as well as increased synthesis of LDL receptors, resulting in an increased clearance of low-density lipoprotein (LDL) from the bloodstream. It has been postulated that there is association between increased risk of neovascular AMD and higher levels of serum cholesterol [33, 34]. Additionally, statins exhibit action beyond lipid-lowering activity in the prevention of atherosclerosis. Researchers hypothesise that statins prevent cardiovascular disease via four proposed mechanisms (all subjects of a large body of biomedical research): improving endothelial function, modulating inflammatory responses, maintaining plaque stability and preventing thrombus formation [35]. Most of these mechanisms are of interest in the pathogenesis of AMD. Nevertheless, recent evaluations of the impact of statin use on the incidence of advanced AMD and its components, choroidal neovascularisation (wet AMD) and geographic atrophy (dry AMD), showed that there is no consistent strong protective effect of statins on the development of advanced AMD among patients with bilateral large drusen [36].
Cyclooxygenase-inhibitors
Cyclooxygenase (COX) is an enzyme that is responsible for formation of important biological mediators called prostanoids like prostaglandins, prostacyclin and thromboxane. Pharmacological inhibition of COX can provide relief from the symptoms of inflammation and pain. Since inflammation is believed to be a part of the AMD pathogenesis puzzle, COX inhibitors may have a therapeutic effect. Nepafenacfor for example is a potent nonsteroidal antiinflammatory drug that rapidly penetrates the eye following topical ocular administration. In the eye, nepafenac is converted to amfenac, which has unique time-dependent inhibitory properties for COX-1 and COX-2. Amfenac treatment significantly reduced retinal prostanoid production and neovascularisation in an in vitro experiment. Therefore nepafenac appears to be a promising advancement in the development of therapies for neovascular eye diseases [37].
COX-inhibitors influence also the proliferation and differentiation of endothelial cells which could probably have another effect especially on neovascular AMD. The results of ongoing studies are still missing.
Complement inhibition
The complement system is a biochemical cascade that helps the ability of antibodies to clear pathogens from an organism. It consists of a number of small proteins found in the blood, generally synthesised by the liver, and normally circulating as inactive precursors (pro-proteins). These proteins are binding to specific complement receptors on the cells of the immune system and are triggering specific cell functions, inflammation, and certain immunoregulatory molecules. Since genetic polymorphism has been found in the complement system in AMD patients, research has started to focus on complement inhibition. Especially complement factor 3 (C3) plays a central role in the activation of complement system. Its activation is required for both classical and alternative complement activation pathways and is therefore a classical target for inhibition of the complement system [38, 39].
Personalised Medicine
Wet AMD treatment
Wet (neovascular) AMD representing a late stage of the disease is nowadays efficiently treated with anti-VEGF therapy in most of the cases [40]. This treatment is performed with an intravitreal injection which often has to be repeated. The most serious known risk of treatment, endophthalmitis, although rare, is always a possibility. Anti-VEGF treatment does not represent a cure of this chronic disease, but helps to transform the more aggressive wet AMD into a dry form. Therefore the goal of the treatment is not healing but stabilisation and prevention of vision loss but also improvement in best-corrected visual acuity. Although inhibition of vascular endothelial growth factor with anti-VEGF has demonstrated efficacy and safety in the treatment of neovascular AMD, novel and combination (for example anti-VEGF and photodynamic therapy) treatments targeting different mechanisms that play a role in CNV development currently are being investigated. Data from these clinical trials will increase our knowledge of the pathogenesis of AMD and likely provide additions to the treatment armamentarium for improving vision and quality of life in patients with AMD [41].
Dry AMD treatment
Regarding early stages of AMD and dry AMD there is at the present time insufficient evidence in the literature to recommend routine nutritional supplementation in healthy adults for primary prevention of AMD. However, patients with intermediate risk of AMD or advanced AMD in one eye should consider taking AREDS-type supplements [27]. Observational studies have also suggested benefit from increased dietary intake of macular xanthophylls and omega-3 fatty acids. These are currently being evaluated prospectively in a randomised controlled clinical trial, the AREDS2 [42].
Concluding remarks and outlook
In summary, AMD is a leading cause of vision loss in people over the age of 60 with a prevalence that continues to rise. Although treatments for AMD were once limited, with disappointing clinical results, new treatments have emerged, which have improved prognostic outcome [43]. Remarkable advances in our understanding of the genetic and biological foundations of this disease were derived from a recent convergence of scientific and clinical data. Collectively, this wealth of information has provided a drive for the development of powerful tools to accurately diagnose disease risk and progression and complement-based therapeutics that will ultimately delay or prevent AMD [44]. In the near future we will have several therapeutic options for treatment of AMD at different stages and therefore personalising more and more the treatment.
References
Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology. 1995;102:205–10.
Vingerling JR, Klaver CC, Hofman A, de Jong PT. Epidemiology of age-related maculopathy. Epidemiol Rev. 1995;17:347–60.
Tomany SC, Klein R, Klein BE. The relationship between iris color, hair color, and skin sun sensitivity and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1526–33.
Bockelbrink A, Roll S, Ruether K, Rasch A, Greiner W, Willich SN. Cataract surgery and the development or progression of age-related macular degeneration: a systematic review. Surv Ophthalmol. 2008;53:359–67.
Holz FG, Sheraidah G, Pauleikhoff D, Bird AC. Analysis of lipid deposits extracted from human macular and peripheral Bruch’s membrane. Arch Ophthalmol. 1994;112:402–6.
Klein R, Klein BE, Moss SE. Diabetes, hyperglycemia, and age-related maculopathy. The beaver dam eye study. Ophthalmology. 1992;99:1527–34.
Young RW. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988;32:252–69.
Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology. 2001;108:697–704.
Evans JR, Fletcher AE, Wormald RP. 28, 000 Cases of age related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. Br J Ophthalmol. 2005;89:550–3.
Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80.
Chakravarthy U, Augood C, Bentham GC, de Jong PT, Rahu M, Seland J, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology. 2007;114:1157–63.
Esparza-Gordillo J, Soria JM, Buil A, Almasy L, Blangero J, Fontcuberta J, et al. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56:77–82.
Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:562–4.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res. 2001;20:227–53.
Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol. 1997;123:199–206.
Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch Ophthalmol. 1998;116:1646–51.
Yates JR, Moore AT. Genetic susceptibility to age related macular degeneration. J Med Genet. 2000;37:83–7.
Scholl HP, Fleckenstein M, Charbel IP, Keilhauer C, Holz FG, Weber BH. An update on the genetics of age-related macular degeneration. Mol Vis. 2007;13:196–205.
Sadaba LM, Fernandez-Robredo P, Rodriguez JA, Garcia-Layana A. Antioxidant effects of vitamins C and E, multivitamin-mineral complex and flavonoids in a model of retinal oxidative stress: the ApoE-deficient mouse. Exp Eye Res. 2008;86:470–9.
Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34.
O'Connell E, Neelam K, Nolan J, Au Eong KG, Beatty S. Macular carotenoids and age-related maculopathy. Ann Acad Med Singapore. 2006;35:821–30.
Spaide RF, Ho-Spaide WC, Browne RW, Armstrong D. Characterization of peroxidized lipids in Bruch’s membrane. Retina. 1999;19:141–7.
Hambidge KM, Krebs NF, Westcott JE, Miller LV. Changes in zinc absorption during development. J Pediatr. 2006;149:S64–8.
Hambidge KM, Miller LV, Tran CD, Krebs NF. Measurements of zinc absorption: application and interpretation in research designed to improve human zinc nutriture. Int J Vitam Nutr Res. 2005;75:385–93.
Karcioglu ZA. Zinc in the eye. Surv Ophthalmol. 1982;27:114–22.
(2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol;119:1417–1436.
Coleman H, Chew E. Nutritional supplementation in age-related macular degeneration. Curr Opin Ophthalmol. 2007;18:220–3.
Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73.
Sangiovanni JP, Agron E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, et al. {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the age-related eye disease study. Am J Clin Nutr. 2009;90:1601–7.
Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J Nutr. 1995;125:790S–7.
Lutty G, Grunwald J, Majji AB, Uyama M, Yoneya S. Changes in choriocapillaris and retinal pigment epithelium in age-related macular degeneration. Mol Vis. 1999;5:35.
Tan JS, Mitchell P, Rochtchina E, Wang JJ. Statins and the long-term risk of incident age-related macular degeneration: the blue mountains eye study. Am J Ophthalmol. 2007;143:685–7.
Tan JS, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the blue mountains eye study. Ophthalmology. 2007;114:1143–50.
Furberg CD. Natural statins and stroke risk. Circulation. 1999;99:185–8.
Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y. Statin use and the incidence of advanced age-related macular degeneration in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2009;116:2381–5.
Yanni SE, Clark ML, Yang R, Bingaman DP, Penn JS. The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res Bull. 2010;81:310–9.
McKay GJ, Dasari S, Patterson CC, Chakravarthy U, Silvestri G. Complement component 3: an assessment of association with AMD and analysis of gene-gene and gene-environment interactions in a Northern Irish cohort. Mol Vis. 2010;16:194–9.
Scholl HP, Fleckenstein M, Fritsche LG, Schmitz-Valckenberg S, Gobel A, Adrion C, et al. CFH, C3 and ARMS2 are significant risk loci for susceptibility but not for disease progression of geographic atrophy due to AMD. PLoS One. 2009;4:e7418.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Do DV. Antiangiogenic approaches to age-related macular degeneration in the future. Ophthalmology. 2009;116:S24–6.
Krishnadev N, Meleth AD, Chew EY. Nutritional supplements for age-related macular degeneration. Curr Opin Ophthalmol. 2010.
Prasad PS, Schwartz SD, Hubschman JP. Age-related macular degeneration: Current and novel therapies. Maturitas 2010.
Gehrs KM, Jackson JR, Brown EN, Allikmets R, Hageman GS. Complement, age-related macular degeneration and a vision of the future. Arch Ophthalmol. 2010;128:349–58.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hasler, P.W., Flammer, J. Predictive, preventive and personalised medicine for age-related macular degeneration. EPMA Journal 1, 245–251 (2010). https://doi.org/10.1007/s13167-010-0017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-010-0017-2