Abstract
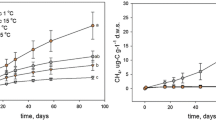
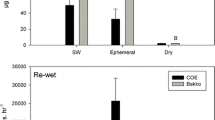
The Maximum Power Principle (MPP) — a theoretical construct that argues that systems develop to maximize energy throughput, or power — is the subject of few empirical studies. We used the MPP to explore the thermodynamic basis for microbial processes and greenhouse gas fluxes in high latitude peat soils. Increasing temperatures cause extensive areas of permafrost degradation, which can lead to wetland formation, though permafrost degradation and aggradation can be cyclical under the right conditions. Differential ecosystem responses to permafrost degradation offer an opportunity to use the unifying approach of thermodynamics. We used adenosine triphosphate (ATP) production in peat soils as a soil-relevant proxy for power to test the MPP along a chronosequence of wetlands with time following permafrost degradation. We conducted soil incubation experiments and measured production rates of CO2, methane (CH4), nitrous oxide (N2O), and ATP. ATP production was significantly lower (p < 0.05) in the young bog soils compared to the undisturbed permafrost bog soils; rates in the older bog soils were not different from either site. Our results suggest that power output increased following recovery from permafrost thaw. A unifying vantage point provided by thermodynamics may be useful in other investigations of wetland ecosystems with unpredictable responses to disturbance.





Similar content being viewed by others
References
Amend JP, Plyasunov AV (2001) Carbohydrates in thermophile metabolism: calculation of the standard molal thermodynamic properties of aqueous pentoses and hexoses at elevated temperatures and pressures. Geochimica et Cosmochimica Acta 65:3901–3917. doi:10.1016/S0016-7037(01)00707-4
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiology Review 25:175–243. doi:10.1111/j.1574-6976.2001.tb00576.x
Cai TT, Montague CL, Davis JS (2006) The maximum power principle: an empirical investigation. Ecologocal Modelling 190:317–335. doi:10.1016/j.ecolmodel.2005.04.022
Camill P, Lynch JA, Clark JS et al (2001) Changes in biomass, aboveground net primary production, and peat accumulation following permafrost thaw in the boreal peatlands of Manitoba, Canada. Ecosystems 4:461–478. doi:10.1007/s10021-001-0022-3
Chambers JQ, Tribuzy ES, Toledo LC et al (2004) Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecological Applications 14:72–88. doi:10.1890/01-6012
Chapin FS, Sturm M, Serreze MC et al (2005) Role of land-surface changes in Arctic summer warming. Science 310:657–660. doi:10.1126/science.1117368
Chapman EJ, Childers DL, Vallino JJ (2016) How the second law of thermodynamics has informed ecosystem ecology through its history. Bioscience 66:27–39
DeLong JP (2008) The maximum power principle predicts the outcomes of two-species competition experiments. Oikos 117:1329–1336
Dilly O, Nannipieri P (2001) Response of ATP content, respiration rate and enzyme activities in an arable and a forest soil to nutrient additions. Biology and Fertility of Soils 34:64–72. doi:10.1007/s003740100375
Duddleston KN, Kinney MA, Kiene RP, Hines ME (2002) Anaerobic microbial biogeochemistry in a northern bog: acetate as a dominant metabolic end product. Global Biogeochemical Cycles 16:11–1–11–9. doi:10.1029/2001GB001402
Eilers KG, Debenport S, Anderson S, Fierer N (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biology & Biochemistry 50:58–65. doi:10.1016/j.soilbio.2012.03.011
Fillingame RH (1999) Molecular rotary motors. Science 286:1687–1688. doi:10.1126/science.286.5445.1687
Fioretto A, Papa S, Pellegrino A, Ferrigno A (2009) Microbial activities in soils of a Mediterranean ecosystem in different successional stages. Soil Biology & Biochemistry 41:2061–2068. doi:10.1016/j.soilbio.2009.07.010
Fisher SG, Likens GE (1972) Stream ecosystems: organic energy budget. Bioscience 22:33–35
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecology Application 1:182–195. doi:10.2307/1941811
Hall SJ, Huber D, Grimm NB (2008) Soil N2O and NO emissions from an arid, urban ecosystem. Journal of Geophysical Research 113:G01016. doi:10.1029/2007JG000523
Hartmann M, Lee S, Hallam SJ, Mohn WW (2009) Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environmental Microbiology 11:3045–3062. doi:10.1111/j.1462-2920.2009.02008.x
Inubushi K, Brookes PC, Jenkinson DS (1989) Adenosine 5′-triphosphate and adenylate energy charge in waterlogged soil. Soil Biology & Biochemistry 21:733–739. doi:10.1016/0038-0717(89)90072-2
Itoh H, Takahashi A, Adachi K et al (2004) Mechanically driven ATP synthesis by F1-ATPase. Nature 427:465–468. doi:10.1038/nature02212
Jenkinson D, Oades JM (1979) A method for measuring adenosine triphosphate in soil. Soil Biology & Biochemistry 11:193–199
Johnstone JF, Chapin FS, Hollingsworth TN et al (2010) Fire, climate change, and forest resilience in interior Alaska. Canadian Journal of Forest Research 40:1302–1312. doi:10.1139/X10-061
Jorgenson MT, Racine CH, Walters JC, Osterkamp TE (2001) Permafrost degradation and ecological changes associated with a warming climate in Central Alaska. Climate Change 48:551–579
Kane ES, Chivers MR, Turetsky MR et al (2013) Response of anaerobic carbon cycling to water table manipulation in an Alaskan rich fen. Soil Biology & Biochemistry 58:50–60. doi:10.1016/j.soilbio.2012.10.032
Kasischke ES, Verbyla DL, Rupp TS et al (2010) Alaska’s changing fire regime — implications for the vulnerability of its boreal forests. Canadian Journal of Forest Research 40:1313–1324. doi:10.1139/X10-098
Larowe DE, Helgeson HC (2007) Quantifying the energetics of metabolic reactions in diverse biogeochemical systems: electron flow and ATP synthesis. Geobiology 5:153–168. doi:10.1111/j.1472-4669.2007.00099.x
Lotka AJ (1922) Contribution to the energetics of evolution. Proceeding of the National Academy of Science of the United State America 8:147–151
Metje M, Frenzel P (2005) Effect of temperature on anaerobic ethanol oxidation and methanogenesis in acidic peat from a northern wetland. Applied and Environmental Microbiology 71:8191–8200. doi:10.1128/AEM.71.12.8191-8200.2005
Myers-Smith IH, Forbes BC, Wilmking M et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environmental Research Letters 6:045509. doi:10.1088/1748-9326/6/4/045509
Odum EP (1969) The strategy of ecosystem development. Science 164:262–270
Odum HT, Pinkerton RC (1955) Time’s speed regulator: the optimum efficiency for maximum power output in physical and biological systems. American Scientist 43:331–343
Osterkamp TE, Viereck L, Shur Y et al (2000) Observations of thermokarst and its impact on boreal forests in Alaska, U.S.a. Arctic, Antarctic, and Alpine Research 32:303–315. doi:10.2307/1552529
Redmile-Gordon M, White RP, Brookes PC (2011) Evaluation of substitutes for paraquat in soil microbial ATP determinations using the trichloroacetic acid based reagent of Jenkinson and Oades (1979. Soil Biology and Biochemistry 43:1098–1100. doi:10.1016/j.soilbio.2011.01.007
Schuur EA, Bockheim J, Canadell JG et al (2008) Vulnerability of permafrost carbon to climate change: implications for the global carbon cycle. Bioscience 58:701–714
Shur YL, Jorgenson MT (2007) Patterns of permafrost formation and degradation in relation to climate and ecosystems. Permafrost and Periglacial Processes 18:7–19. doi:10.1002/ppp.582
Stock D, Leslie AGW, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286:1700–1705. doi:10.1126/science.286.5445.1700
Tape K, Sturm M, Racine C (2006) The evidence for shrub expansion in northern Alaska and the pan-Arctic. Global Change Biology 12:686–702. doi:10.1111/j.1365-2486.2006.01128.x
Turetsky MR, Treat CC, Waldrop MP et al (2008) Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. Journal of Geophysical Research 113:G00A10. doi:10.1029/2007JG000496
Vinther FP, Eiland F, Lind A-M, Elsgaard L (1999) Microbial biomass and numbers of denitrifiers related to macropore channels in agricultural and forest soils. Soil Biology and Biochemistry 31:603–611. doi:10.1016/S0038-0717(98)00165-5
Waldrop MP, Harden JW (2008) Interactive effects of wildfire and permafrost on microbial communities and soil processes in an Alaskan black spruce forest. Global Change Biology 41:2591–2602. doi:10.1111/j.1365-2486.2008.01661.x
Acknowledgments
EJC received support from the Graduate Student and Professional Association and the School of Life Sciences at Arizona State University for sampling and travel. Additional support was provided by Central Arizona-Phoenix Long-Term Ecological Research Program (NSF Grant No. 1027188). Support was also provided to DLC by the National Science Foundation through the Urban Sustainability Research Coordination Network (NSF Grant No. 1140070). ELS acknowledges support from NSF Grant No. 1123649. We thank the APEX team, Mark Waldrop for field and intellectual support, and Brian St. Clair for help with organic ion analyses. We thank two anonymous reviewers that provided comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chapman, E.J., Childers, D.L., Shock, E.L. et al. A Thermodynamic Analysis of Soil Ecosystem Development in Northern Wetlands. Wetlands 36, 1143–1153 (2016). https://doi.org/10.1007/s13157-016-0833-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-016-0833-9