Abstract
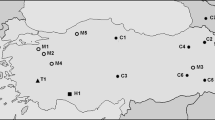
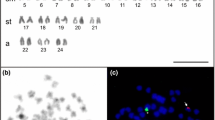
The As-51 satellite DNA is a transposon-like sequence formerly described for arthropods and physically identifiable by fluorescent in situ hybridization. In the present work, we describe the occurrence of this sequence, as well the C-banding and karyotype composition, in populations of the group Astyanax fasciatus from Mogi-Guaçu (Araras-SP), Paranapanema (Angatuba and Pilar do Sul-SP), Ribeira de Iguape (Sete Barras-SP) and Tietê (Indaiatuba and Salesópolis-SP) river basins. The specimens from Sete Barras (10 M + 20SM + 12ST + 6A) and Araras (8 M + 22SM + 12ST + 6A) have 2n = 48 chromosomes. The samples from Angatuba, Pilar do Sul and Indaiatuba presented 2n = 46 chromosomes (12 M + 20SM + 10ST + 4A). The individuals collected in Salesópolis showed three cytotypes, bearing 2n = 46 (12 M + 20SM + 10ST + 4A), 2n = 48 (8 M + 22SM + 12ST + 6A) and 2n = 50 (8 M + 16SM + 14ST + 12A). C-banding revealed large heterochromatic blocks at terminal chromosomal regions in all populations and/or cytotypes. All analyzed populations have conspicuous blocks carrying the As-51 satellite DNA, although the number of chromosomes bearing this repetitive sequence was variable among them. Such differences were not related to the diploid number of individuals, but rather to a biogeographic pattern. Aspects of the karyotype evolution and distribution of this sequence in distinct populations are discussed.




Similar content being viewed by others
References
Ab’Saber, N. A. (1957). O problema das conexões antigas e da separação da drenagem do Paraíba e Tietê. Boletim Paulista de Geografia, 26, 38–49.
Abel, L. D. S., Mantovani, M., & Moreira-Filho, O. (2006). Chromosomal distribution of the As-51 satellite DNA in two species complexes of the genus Astyanax (Pisces, Characidae). Genetics and Molecular Biology, 29, 448–452.
Almeida, F. F. M., & Carneiro, C. D. R. (1998). Origem e evolução da Serra do Mar. Revista Brasileira de Geociências, 28, 135–150.
Bizerril, C. R. S. F. (1994). Análise taxonômica e biogeográfica da ictiofauna de água doce do leste brasileiro. Acta Biologica Leopoldensia, 16, 51–80.
Castro, R. M. C. (1999). Evolução da ictiofauna de riachos sul-americanos: padrões gerais e possíveis processos causais. In E. P. Caramashi, R. Mazzoni, & P. R. Peres-Neto (Eds.), Ecologia de Peixes de Riachos. Rio de Janeiro: PPGE-UFRJ.
Centofante, L., Bertollo, L. A. C., Justi, A. J., & Moreira-Filho, O. (2003). Correlation of chromosomal and morphologic characters in two Astyanax species (Teleostei: Characidae). Ichthyological Exploration of Freshwaters, 14, 361–368.
Fernandes, C. A., & Martins-Santos, I. C. (2004). Cytogenetic studies in two populations of Astyanax altiparanae (Pisces, Characiformes). Hereditas, 141, 328–332.
Gold, J. R., Li, C., Shipley, N. S., & Powers, P. K. (1990). Improved methods for working with fish chromosomes with a review of metaphase chromosome banding. Journal of Fish Biology, 37, 563–575.
Gross, M. C., Schneider, C. E., Matiello, M. C. A., Leite, M. L., Bertollo, L. A. C., & Artoni, R. F. (2004). Population structure, fluctuating asymmetry and genetic variability in an endemic and highly isolated Astyanax fish Population (Characidae). Genetics and Molecular Biology, 27, 529–535.
Jin, S. N., & Toledo, V. (1975). Citogenética de Astyanax fasciatus e Astyanax bimaculatus (Characidae, Tetragonopterinae). Ciência e Cultura, 27, 1122–1124.
Justi, A.J. (1993). Caracterização cariotípica de populações de Astyanax fasciatus (Cuvier, 1819), Pisces, Characidae, em três bacias hidrográficas. MSc Thesis, Federal University of São Carlos.
Kantek, D. L. Z., Vicari, M. R., Peres, W. A. M., Cestari, M. M., Artoni, R. F., Bertollo, L. A. C., et al. (2009). Chromosomal location and distribution of As51 satellite DNA in five species of the genus Astyanax (Teleostei, Characidae, Incertae sedis). Journal of Fish Biology, 75, 408–421.
Kavalco, K. F., & Almeida-Toledo, L. F. (2007). Molecular cytogenetics of blind mexican tetra and comments on the karyotypic characteristics of genus Astyanax (Teleostei, Characidae). Zebrafish, 4, 103–111.
Kavalco, K. F., & Moreira-Filho, O. (2003). Cytogenetical analyses in four species of the genus Astyanax (Pisces, Characidae) from Paraíba do Sul river basin. Caryologia, 56, 453–461.
Kavalco, K. F., Pazza, R., Bertollo, L. A. C., & Moreira-Filho, O. (2007). Satellite DNA sites of four species of the genus Astyanax (Teleostei, Characiformes). Genetics and Molecular Biology, 30, 329–335.
Kavalco, K. F., Brandão, K. O., Pazza, R., & Almeida-Toledo, L. F. (2009). Astyanax hastatus Myers, 1928 (Teleostei, Characidae): a new species complex within the genus Astyanax? Genetics and Molecular Biology, 32, 477–483.
Kavalco, K. F., Pazza, R., & Almeida-Toledo, L. F. (2009). Astyanax bockmanni Vari and Castro, 2007: an ambiguous karyotype in the Astyanax genus. Genetica, 136, 135–139.
Kavalco, K. F., Pazza, R., & Almeida-Toledo, L. F. (2010). Molecular cytogenetics of Astyanax ribeirae (Teleostei, Characidae), an endemic characin of the Atlantic rainforest. The Nucleus, 53, 51–54.
Kavalco, K.F., Brandão, K.O., Garcia, C., Pazza, R., & Almeida-Toledo, L.F. (2011). Comparative cytogenetics and molecular phylogeography in the group Astyanax altiparanae - Astyanax aff. bimaculatus (Teleostei, Characidae). Cytogenetic and Genome Research, 134(2), 108-119. doi:10.1159/000325539.
Levan, A., Fredga, K., & Sandberg, A. A. (1964). Nomenclature for centromeric position on chromosomes. Hereditas, 52, 201–220.
Mantovani, M., Abel, L. D. S., Mestriner, C. A., & Moreira-Filho, O. (2000). Accentuated polymorphism of heterochromatin and nucleolar organizer regions in Astyanax scabripinnis (Pisces, Characidae): tools for understanding karyotypic evolution. Genetica, 109, 161–168.
Mantovani, M., Abel, L. D. S., & Moreira-Filho, O. (2004). Evidence of the differentiated structural arrangement of constitutive heterochromatin between two populations of Astyanax scabripinnis (Pisces, Characidae). Genetics and Molecular Biology, 27, 536–542.
Melo, F. A. G. (2001). Revisão taxonômica das espécies do gênero Astyanax Baird e Girard, 1854 (Teleostei: Characiformes: Characidae) da região da Serra dos Órgãos, Rio de Janeiro. Arquivos do Museu Nacional, 59, 1–46.
Mestriner, C. A., Galetti, P. M., Jr., Valentini, S. R., Ruiz, I. R. G., Abel, L. D. S., Moreira-Filho, O., et al. (2000). Structural and functional evidence that a B chromosome in the characid fish Astyanax scabripinnis is an isochromosome. Heredity, 85, 1–9.
Moreira-Filho, O., & Bertollo, L. A. C. (1991). Astyanax scabripinnis (Pisces, Characidae): a species complex. Genetics and Molecular Biology, 14, 331–357.
Moreira-Filho, O., & Buckup, P. A. (2005). A poorly known case of watershed transposition between the São Francisco and upper Paraná river basins. Neotropical Ichthyology, 3, 449–452.
Morelli, S., Bertollo, L. A. C., Foresti, F., Moreira-Filho, O., & Toledo-Filho, S. A. (1983). Cytogenetic considerations on the genus Astyanax (Pisces, Characidae). I. Karyotypic variability. Caryologia, 36, 235–244.
Paiva, M. P. (1982). As grandes represas do Brasil. Brasília: Editerra.
Pazza, R., Kavalco, K. F., & Bertollo, L. A. C. (2006). Chromosome polymorphism in Astyanax fasciatus (Teleostei, Characidae). 1 - Karyotypic analysis, Ag-NORs and mapping of the 18 S and 5 S ribosomal genes in sympatric karyotypes and their possible hybrid forms. Cytogenetic and Genome Research, 112, 313–319.
Pazza, R., Kavalco, K. F., Prioli, S. M. A. P., Prioli, A. J., & Bertollo, L. A. C. (2007). Chromosome polymorphism in Astyanax fasciatus (Teleostei, Characidae). 3 – Analysis of the RAPD and ISSR molecular markers. Biochemical Systematics and Ecology, 35, 841–851.
Pazza, R., Kavalco, K. F., & Bertollo, L. A. C. (2008). Chromosome polymorphism in Astyanax fasciatus (Teleostei, Characidae). 2 - Gene mapping of satellite DNA. Cytogenetic and Genome Research, 122, 61–66.
Pazza, R., Kavalco, S. A. F., Penteado, P. R., Kavalco, K. F., & Almeida-Toledo, L. F. (2008). The species complex Astyanax fasciatus Cuvier, 1819 (Teleostei, Characiformes): a multidisciplinary approach. Journal of Fish Biology, 72, 2002–2010.
Peres, W. A. M., Buckup, P. A., Kantek, D. L. Z., Bertollo, L. A. C., & Moreira-Filho, O. (2009). Chromosomal evidence of downstream dispersal of Astyanax fasciatus (Characiforems, Characidae) associated with river shed interconnection. Genetica, 137, 305–311.
Pinkel, D., Straume, T., & Gray, J. W. (1986). Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proceedings of National Academy of Science, 83, 2934–2938.
Ribeiro, A. C. (2006). Tectonic history and the biogeography of the freshwater fishes from the coastal drainages of eastern Brazil: an example of faunal evolution associated with a divergent continental margin. Neotropical Ichthyology, 4, 225–246.
Strecker, U., Faundez, W. H., & Wilkens, H. (2004). Phylogeography of surface and cave Astyanax (Teleostei) from Central and North America based on cytochrome b sequence data. Molecular Phylogenetics and Evolution, 33, 469–481.
Sumner, A. T. (1972). A simple technique for demonstrating centromeric heterocromatin. Experimental Cell Research, 75, 304–306.
Vicari, M. R., Artoni, R. F., Moreira-Filho, O. & Bertollo, L. A. C. (2008). Colocalization of repetitive DNAs and silencing of major rRNA genes. A case report of the fish Astyanax janeiroensis. Cytogenetic and Genome Research, 122, 67–72. doi:10.1159/000151318.
Acknowledgments
The authors wish to thank Malabarba LR and Bertaco V, for the taxonomical identification; Lopes CE for helping with the samples; Smarzaro RS for helping with the GIS software; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavalco, K.F., Pazza, R., de Oliveira Brandão, K. et al. Biogeographic patterns in the chromosomal distribution of a satellite DNA in the banded tetra Astyanax fasciatus (Teleostei: Characiformes). Org Divers Evol 13, 67–76 (2013). https://doi.org/10.1007/s13127-012-0100-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-012-0100-8