Abstract
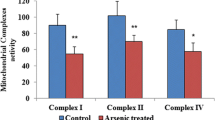
The present investigation evaluates the changes in the levels of antioxidant enzymes, lipid peroxidation (LPO), and protein carbonyl content (PCC) in brain mitochondria following thiamine deficiency (TD). The study was carried out on Mus musculus allocated into three groups, namely control and thiamine-deficient group for 8 (TD 8) and 10 (TD 10) days. The LPO was measured in terms of reduced glutathione (GSH) and thiobarbituric acid reactive substance (TBARS). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured biochemically. A significant increase in the TBARS (p < 0.0001) and PCC (p < 0.001) levels in group II (TD 8) and group III (TD 10) animals was observed in comparison to controls. The GSH levels were found to be reduced in both the treated groups compared to the control. A significant reduction in the activities of SOD was also observed in group II (p < 0.01) and group III (p < 0.0001) animals in comparison to the control. Enzymatic activities of CAT (p < 0.001) and GPx (p < 0.05) were found to be significantly reduced in group III (TD 10) in comparison to the control. In conclusion, reduction in the activities of antioxidant enzymes as well as an increase in LPO and PCC following TD implies oxidative stress in brain mitochondria that may further leads to neurodegeneration.






Similar content being viewed by others
References
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Bist R, Bhatt DK (2010) Augmentation of cholinesterases and ATPase activities in the cerebellum and pons-medulla oblongata, by a combination of antioxidants (resveratrol, ascorbic acid, alpha-lipoic acid and vitamin E), in acutely lindane intoxicated mice. J Neurol Sci 296:83–87
Bist R, Misra S, Bhatt DK (2010) Inhibition of lindane-induced toxicity using alpha-lipoic acid and vitamin E in the brain of Mus musculus. Protoplasma 243:49–53
Blass JP (2001) Brain metabolism and brain disease: is metabolic deficiency the proximate cause of Alzheimer dementia? J Neurosci Res 66(5):851–856
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Bowling AC, Schulz JB, Brown RH Jr, Beal MF (1993) Superoxide dismutase activity, oxidative damage and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem 61:2322–2325
Butterworth RF, Giguere JF, Besnard AM (1985) Activities of thiamine-dependent enzymes in two experimental models of thiamine deficiency encephalopathy: 1. The pyruvate dehydrogenase complex. Neurochem Res 10:1417–1428
Calingasan NY, Park LC, Calo LL, Trifiletti RR, Gandy SE, Gibson GE (1998) Induction of nitric oxide synthase and microglial responses precede selective cell death induced by chronic impairment of oxidative metabolism. Am J Pathol 153(2):599–610
Calvin HI, Medvedovsky C, Worgul BW (1986) Near-total glutathione depletion and age specific cataracts induced buthionine by sulfoximine in mice. Science (Washington, DC) 233:553–555
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Chavko M, Harabin AL (1996) Regional lipid peroxidation and protein oxidation in rat brain after hyperberic oxygen exposure. Free Radic Biol Med 20:973–978
Choi S, Park KA, Lee HJ, Park MS, Lee JH, Park KC, Kim M, Seung-Hoon L, Jeong-Sun S, Byung-Woo Y (2005) Expression of Cu/Zn SOD protein is suppressed in hsp 70.1 knockout mice. J Biochem Mol Biol 38:111–114
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clinica Chimica Acta 329:23–38
Desjardins P, Butterworth RF (2005) Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol 31:17–25
Dhindsa RH, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ferreiro E, Baldeiras I, Ferreira IL, Costa RO, Rego AC, Pereira CF, Oliveira CR (2012) Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer’s disease: from pathogenesis to biomarkers. Int J Cell Biol 2012:1–23
Floor E, Wetzel MG (1998) Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazene method assay. J Neurochem 70:268–275
Gibson GE, Ksiezak-Reding H, Sheu KF, Mykytyn V, Blass JP (1984) Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res 9(6):803–814
Gibson GE, Zhang H (2002) Interactions of oxidative stress with thiamine homeostasis promote neurodegeneration. Neurochem Int 40:493–504
Gioda CR et al (2010) Cardiac oxidative stress is involved in heart failure induced by thiamine deprivation in rats. Am J Physiol Heart Circ Physiol 298:H2039–H2045
Gorman GS, Taylor RW (2011) Mitochondrial DNA abnormalities in ophthalmological disease. Saudi J Ophthalmol 25(4):395–404
Halliwell B, Gutteridge JMC (1985) Oxygen radicals and nervous system. Trends Neurosci 8:22–26
Jhala SS, Hazell AS (2011) Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int 58:248–260
Keeney PM, Xie J, Capaldi RA, Bennett JP (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci 19:256–264
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Madrigal JL, Olivenza R, Moro MA, Lizasoin I, Lorenzo P, Rodrigo J, Leza JC (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. J Neuropsychopharmacol 24:420–429
Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L (2006) Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alz Dis 10:59–73
Manczak M, Park BS, Jung Y, Reddy PH (2004) Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromol Med 5:147–162
Manimaran A, Chellappan PR (2009) Activities of antioxidant enzyme and lipid peroxidation in ovarian cancer patients. Acad J Can Res 2(2):68–72
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mohandas J, Marshal J, Duggin G, Horvath JS, Tiller D (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidney. Cancer Res 44:5086–5091
Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, Zhu X, Perry G (2007) Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol 66:525–532
Ohkawa H, Ohshi N, Yagi K (1979) Assay or lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paliwal R, Sharma V, Pracheta, Sharma SH (2011) Hepatoprotective and antioxidant potential of Moringa oleifera pods against DMBA-induced hepatocarcinogenesis in male mice. Int J Drug Dev Res 3(2):128–138
Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, Castellani RJ, Atwood CS, Aliev G, Sayre LM, Takeda A, Smith MA (2002) Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med 33:1475–1479
Raja S, Ahamed HN, Kumar V, Mukherjee K, Bandyopadhyay A, Mukherjee PK (2007) Exploring the effect of Cytisus scoparius on markers of oxidative stress in rats. Iranian J Pharmaco Thera 6(1):15–21
Reznick AZ, Packer L (1993) Free radicals and antioxidants in muscular neurological diseases and disorders. In: Poli G, Albano E, Dianzani MU (eds) Free radicals: from basic science to medicine. Birkhauser, Basel, pp 425–437
Shangari N, Bruce WR, Poon R, Obrien PJ (2003) Toxicity of glyoxals—role of oxidative stress, metabolic detoxification and thiamine deficiency. Biochem Soc Trans 31(Pt 6):1390–1393
Sharma N, Garg V, Paul A (2010) Antihyperglycemic, antihyperlipidemic and antioxidative potential of Prosopis cineraria bark. Indian J Clin Biochem 25(2):193–200
Sies H, Cadenas E (1985) Oxidative stress: damage to intact cells and organs. Philos Trans R Soc London B 311:617–631
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277:44784–44790
Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F (2003) The ala 16 Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenet 13:145–157
Tang J, Yan H, Zhuang S (2012) Inflammation and oxidative stress in obesity-related glomerulopathy. Int J Nephrol 2012:1–11
Tirmenstein MA, Nicholls-Grzemski FA, Zhang JG, Fariss MW (2000) Glutathione depletion and the production of reactive oxygen species in isolated hepatocyte suspensions. Chem Biolo Interact 127:201–217
Vermulst M, Bielas JH, Loeb LA (2008) Quantification of random mutation in the mitochondrial genome. Methods 46:263–268
Acknowledgments
We acknowledge Prof. Aditya Shastri, Vice Chancellor, Banasthali University, Rajasthan for providing the suitable facilities to carry out the present investigation in the Department of Bioscience and Biotechnology.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, A., Bist, R. & Bubber, P. Thiamine deficiency induces oxidative stress in brain mitochondria of Mus musculus . J Physiol Biochem 69, 539–546 (2013). https://doi.org/10.1007/s13105-013-0242-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-013-0242-y