Abstract
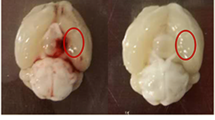
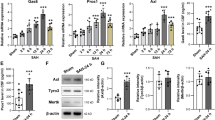
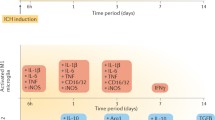
Polarized microglia play a dual (beneficial/detrimental) role in neurological diseases. However, the status and the factors that modulate microglia polarization in intracerebral hemorrhage (ICH) remain unclear. In the present study, we investigated the role of protease-activated receptor-1 (PAR-1, a thrombin receptor) in ICH-induced microglia polarization in mice. Male wild-type (WT) and PAR-1 knockout (PAR-1 KO) mice received an infusion of 30-μL autologous blood or saline into the right basal ganglia. Mice were euthanized at different time points and the brains were used for Western blotting and immunohistochemistry. Some mice had magnetic resonance imaging. We found that ICH induced microglia activation and polarization. M1 phenotypic markers were markedly increased and reached a peak as early as 4 h, remained high at 3 days and decreased 7 days after ICH. M2 phenotypic markers were upregulated later than M1 markers reaching a peak at day 1 and declining by day 7 after ICH. PAR-1 was upregulated after ICH and expressed in the neurons and microglia. ICH induced less brain swelling and neuronal death in PAR-1 KO mice, and this was associated with less M1 polarization and reduced proinflammatory cytokine levels in the brain. In conclusion, these results suggest that polarized microglia occur dynamically after ICH and that PAR-1 plays a role in the microglia activation and polarization.







Similar content being viewed by others
References
Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92(4):463–77. Pubmed Central PMCID: 2991407.
Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63.
van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19(3-4):393–411.
Eyo UB, Wu LJ. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013;2013:456857. Pubmed Central PMCID: 3775394.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. Pubmed Central PMCID: 3719181.
Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4(4):399–418. Pubmed Central PMCID: 2773116.
Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. Pubmed Central PMCID: 3810327.
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44.
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64. Pubmed Central PMCID: 4395497.
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–44. Pubmed Central PMCID: 2788152.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70.
Goerdt S, Politz O, Schledzewski K, Birk R, Gratchev A, Guillot P, et al. Alternative versus classical activation of macrophages. Pathobiology. 1999;67(5-6):222–6.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60(5):717–27.
Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cerebral Blood Flow Metabolism. 2013;33(12):1864–74. Pubmed Central PMCID: 3851898.
Kumar A, Alvarez-Croda DM, Stoica BA, Faden AI, Loane DJ. Microglial/Macrophage Polarization Dynamics following Traumatic Brain Injury. J neurotrauma. 2015. doi:10.1089/neu.2015.4268.
Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. Pubmed Central PMCID: 3251548.
Yabluchanskiy A, Sawle P, Homer-Vanniasinkam S, Green CJ, Motterlini R. Relationship between leukocyte kinetics and behavioral tests changes in the inflammatory process of hemorrhagic stroke recovery. Int J Neurosci. 2010;120(12):765–73.
Xue M, Del Bigio MR. Intracerebral injection of autologous whole blood in rats: time course of inflammation and cell death. Neurosci Lett. 2000;283(3):230–2.
Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–54.
Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407(6801):258–64.
Xue M, Hollenberg MD, Demchuk A, Yong VW. Relative importance of proteinase-activated receptor-1 versus matrix metalloproteinases in intracerebral hemorrhage-mediated neurotoxicity in mice. Stroke. 2009;40(6):2199–204. Epub 2009/04/11. eng.
Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, et al. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 2003;100(22):13019–24. Pubmed Central PMCID: 240737.
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y. Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res. 2014;5(4):472–5. Pubmed Central PMCID: 3962522.
Xi G, Strahle J, Hua Y, Keep RF. Progress in translational research on intracerebral hemorrhage: is there an end in sight? Prog Neurobiol. 2014;115C:45–63. Pubmed Central PMCID: 3961535.
Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100(4):672–8.
Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30(6):1247–55.
Zheng M, Du H, Ni W, Koch LG, Britton SL, Keep RF, et al. Iron-induced necrotic brain cell death in rats with different aerobic capacity. Transl Stroke Res. 2015;6(3):215–23. Pubmed Central PMCID: 4425582.
Okauchi M, Xi G, Keep RF, Hua Y. Tissue-type transglutaminase and the effects of cystamine on intracerebral hemorrhage-induced brain edema and neurological deficits. Brain Res. 2009;1249:229–36. eng.
Schmued LC, Albertson C, Slikker Jr W. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751(1):37–46.
Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–31. Pubmed Central PMCID: 3884550.
Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–605.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69. Pubmed Central PMCID: 2724991.
Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229(2):176–85.
Ni W, Mao S, Xi G, Keep RF, Hua Y. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke; a journal of cerebral circulation. 2016;47:505–11
Zhao H, Garton T, Keep RF, Hua Y, Xi G. Microglia/Macrophage Polarization After Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2015;6(6):407–9. Pubmed Central PMCID: 4628553.
Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev. 2007;56(2):331–45.
McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104(13):5662–7. Pubmed Central PMCID: 1838494.
Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, et al. A dual thrombin receptor system for platelet activation. Nature. 1998;394(6694):690–4.
Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84(1):3–9.
Adams MN, Ramachandran R, Yau MK, Suen JY, Fairlie DP, Hollenberg MD, et al. Structure, function and pathophysiology of protease activated receptors. Pharmacol Ther. 2011;130(3):248–82.
Gieseler F, Ungefroren H, Settmacher U, Hollenberg MD, Kaufmann R. Proteinase-activated receptors (PARs) - focus on receptor-receptor-interactions and their physiological and pathophysiological impact. Cell Communicat signal. 2013;11:86. Pubmed Central PMCID: 3842752.
Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50(4):329–39.
Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–61.
Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161(1-2):113–22.
Acknowledgments
This study was supported by grants NS-073595, NS-079157, NS-084049, NS-090925, NS-091545 and NS-096917 from the National Institutes of Health (NIH), 973 Program-2014CB541600, and by grants 81371336, 81400942, 81400945 from National Natural Science Foundation of China (NSFC) and grants LY13H090005 from Natural Science Foundation of Zhejiang Province (ZJNSF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NSFC, and ZJNSF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Shu Wan, Yingying Cheng, Hang Jin, Dewei Guo, Ya Hua, Richard F. Keep, and Guohua Xi declare that they have no conflict of interest.
Compliance with Ethics Requirements
All institutional and national guidelines for the care and use of laboratory animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplemental Figure I: Wild-type (WT) mice received an intracerebral injection of autologous whole blood or saline. At 24 hours, the brains were used to assess PAR-1 immunoreactivity (A) and protein levels (B) in the ipsilateral basal ganglia. Scale bar = 50 μm; values are means ± S.D.; n = 3 per group, *p < 0.01 vs. other groups. Supplemental Figure II: Immunoreactivity of cluster of differentiation 16 (CD16, A) and inducible nitric oxide synthase (iNOS, B) in the ipsilateral basal ganglia 1 day after injection of 30 μL autologous blood or saline into the right caudate. Scale bar=20μm. Supplemental Figure III: Immunoreactivity and protein levels of cluster of differentiation (CD206, A) and chitinase 3 like protein 3 (YM-1, B) in the ipsilateral basal ganglia 1 day after injection of 30 μL autologous blood or saline into the right caudate. Scale bar=20μm. (PDF 28266 kb)
Rights and permissions
About this article
Cite this article
Wan, S., Cheng, Y., Jin, H. et al. Microglia Activation and Polarization After Intracerebral Hemorrhage in Mice: the Role of Protease-Activated Receptor-1. Transl. Stroke Res. 7, 478–487 (2016). https://doi.org/10.1007/s12975-016-0472-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0472-8