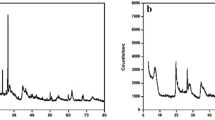
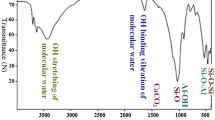
Abstract
This study deals with the use of the natural, low-cost sorbents bentonite and zeolite for the removal of lead from aqueous solutions. The mineral material is from large deposits of bentonite and zeolite that have been discovered recently in Iran. Experimental and modeling data from our kinetic and equilibrium investigations reveal that (1) the pseudo-second-order kinetic model gave the best fit, and (2) the Koble–Corrigan sorption model describes the interaction between Pb(II) and the two mineral materials better than the Freundlich and Langmuir models. However, the sorption of Pb(II) ions by zeolite and bentonite is complex and probably involves several mechanisms. The experimental data show that natural zeolite and bentonite used in this study exhibited a reasonable sorption capacity for Pb(II), and thus may be useful for the immobilization of Pb(II) from polluted sites.






Similar content being viewed by others
References
Aharoni C, Sparks DL, Levinson S, Ravina I (1991) Kinetics of soil chemical reactions: relationships between empirical equations and diffusion models. Soil Sci Soc Am J 55:1307–1312
Campos V (2009) The sorption of toxic elements onto natural zeolite, synthetic goethite and modified powdered block carbon. Environ Earth Sci 59:737–744
Chaari I, Fakhfakh E, Chakroun S, Bouzid J, Boujelben N, Feki M, Rocha F, Jamoussi F (2008) Lead removal from aqueous solutions by a Tunisian smectitic clay. J Hazard Mater B 156:545–551
Chen H, Wang A (2007) Kinetic and isothermal studies of lead ion adsorption onto palygorskite clay. J Colloid Interf Sci 307:309–316
Curkovic L, Stefanovic SC, Filipan T (1997) Metal ion-exchange by natural and modified zeolites. Water Res 31:1379–1382
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Echeverría JC, Zarranz I, Estella J, Garrido JJ (2005) Simultaneous effect of pH, temperature, ionic strength, and initial concentration on the retention of lead on illite. Appl Clay Sci 30:103–115
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Giles CH, Smith D, Huitson A (1947) A general treatment and classification of the solute adsorption isotherm. I. Theor J Colloid Interf Sci 47:755–765
Günay A, Arslankaya E, Tosun I (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371
Gustafsson JP (2006) Visual MINTEQ, 2.50, software manual, KTH, Department of Land and Water Resources Engineering, Stockholm, Sweden. Available at http://www.lwr.kth.se/English/OurSoftware/vminteq/
Hall KR, Eagleton LC, Acrivos A, Vemeulen T (1966) Pore and solid diffusion in fixed bed adsorption under constant pattern condition. Ind Eng Chem Fund 5:213–223
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater B 136:681–689
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Inglezakis VJ, Stylianou MA, Gkantzou D, Loizidou MD (2007) Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 210:248–256
Kabbashi NA, Atieh MA, Al-Mamun A, Mirghami MES, Alam MDZ, Yahya N (2009) Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J Environ Sci 21:539–544
Kalbasi M, Peryea FG, Lindsay WL, Drake SR (1995) Measurement of divalent lead activity in lead arsenate contaminated soils. Soil Sci Soc Am J 59:1274–1280
Koble RA, Corrigan TE (1952) Adsorption isotherms for pure hydrocarbons. Ind Eng Chem Res 44:383–387
Kumar KV, Sivanesan S (2006) Pseudo-second-order kinetic models for safranin sorption onto rice husk: comparison of linear and non-linear regression analysis. Process Biochem 41:1198–1202
Lagergren S (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe. Handlingar 24:1–39
Langmuir I (1918) Adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lao C, Zeledón Z, Gamisans X, Solé M (2005) Sorption of Cd(II) and Pb(II) from aqueous solutions by a low-rank coal (leonardite). Sep Purif Technol 45:79–85
Mathialagan T, Viraraghavan T (2002) Adsorption of cadmium from aqueous solutions by perlite. J Hazard Mater B 94:291–303
Moore DM, Reynolds RC (1989) X-ray diffraction and the identification and analysis of clay minerals. Oxford Univ. Press, New York
Örnek A, Özacar M, Şengil ÍA (2007) Adsorption of lead onto formaldehyde or sulphuric acid treated acorn waste: equilibrium and kinetic studies. Biochem Eng J 37:192–200
Rhoads JW (1986) Cation exchange capacity. In: Page CA (ed) Methods of soil analysis, Part 2. ASA, Madison, WI, pp 149–158
Runping H, Zhang J, Zou W, Shi J, Liu H (2005) Equilibrium biosorption isotherm for lead ion on chaff. J Hazard Mater B 125:266–271
Saha UK, Taniguchi S, Sakurai K (2002) Simultaneous adsorption of cadmium, zinc, and lead on hydroxyaluminum- and hydroxyaluminosilicate-montmorillonite complexes. Soil Sci Soc Am J 66:117–128
Santilan-Medrano J, Jurniaak JJ (1975) The chemistry of lead and cadmium in soil: solid phase formation. Soil Sci Soc Am J 39:851–856
Sen Gupta S, Bhattacharyya KG (2008) Immobilization of Pb(II), Cd(II) and Ni(II) ions on kaolinite and montmorillonite surfaces from aqueous medium. J Environ Manag 87:46–58
Shahwan T, Erten HN (2002) Thermodynamic parameters of Cs+ sorption on natural clays. J Radioanal Nucl Chem 253:115–120
Shirvani M, Shariatmadari H, Kalbasi M, Nourbakhsh F, Najafi B (2006) Sorption of cadmium on palygorskite, sepiolite and calcite: equilibria and organic ligand affected kinetics. Colloid Surf A 287:182–190
Sparks DL (2003) Environmental soil chemistry. Academic Press, San Diego, CA
Strawn DG, Sparks DL (1999) Sorption kinetics of trace elements in soils and soil materials. In: Selim HM, Iskandar I (eds) Fate and transport of heavy metals in the vadose zone. Lewis Publishers, Chelsea, MI, pp 1–28
Stumm W (1992) Chemistry of the solid-water interface. Wiley-InterScience, New York
Trgo M, Perić J (2003) Interaction of the zeolitic tuff with Zn-containing simulated pollutant solutions. J Colloid Interf Sci 260:166–175
Unuabonah EI, Olu-Owolabi BI, Adebowale KO, Ofomaja AE (2007) Adsorption of lead and cadmium ions from aqueous solutions by tripolyphosphate-impregnated kaolinite clay. Colloid Surf A 292:202–211
Wang S, Dong Y, He M, Chen L, Yu X (2009) Characterization of GMZ bentonite and its application in the adsorption of Pb(II) from aqueous solutions. Appl Clay Sci 43:164–171
Wingenfelder U, Nowack B, Furrer G, Schulin R (2005) Adsorption of Pb and Cd by amine-modified zeolite. Water Res 39:3287–3297
Xu D, Tan XL, Chen CL, Wang XK (2008) Adsorption of Pb(II) from aqueous solution to MX-80 bentonite: effect of pH, ionic strength, foreign ions and temperature. Appl Clay Sci 41:37–46
Zhang SQ, Hou WG (2008) Adsorption behavior of Pb (II) on montmorillonite. Colloid Surf A 320:92–97
Zhu SJ, Hou HB, Xue YJ (2008) Kinetic and isothermal studies of lead ion adsorption onto bentonite. Appl Clay Sci 40:171–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamidpour, M., Kalbasi, M., Afyuni, M. et al. Sorption of lead on Iranian bentonite and zeolite: kinetics and isotherms. Environ Earth Sci 62, 559–568 (2011). https://doi.org/10.1007/s12665-010-0547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0547-x