Abstract
Background
Reactive oxygen species (ROS) have been implicated in the turnover of epithelial cells in the rat intestine. The metabolism of ethanol generates ROS, which are implicated in cellular injury, but the levels of lipid peroxidation in intestine in chronic alcoholism are unknown.
Aim
To investigate the effects of ethanol ingestion on lipid peroxidation, and anti- and pro-oxidant enzyme systems in enterocytes across the crypt-villus axis in intestine.
Methods
Wistar rats (90–100 g) were administered 1 mL of 30% ethanol daily for 39 days. Intestinal epithelial cells were isolated in fractions. Malondialdehyde levels, and activities of glutathione-S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD) and catalase were determined in various cell fractions. Incorporation of H3-thymidine into DNA of enterocytes was also determined.
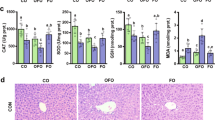
Results
Lipid peroxidation was elevated by two- to threefolds in both villus and crypt cells in ethanol-fed animals compared to controls. The activities of GST and GR were four- to six-folds higher in villus tip cells compared to crypt base cells. The activities of SOD and catalase were five- to seven-fold higher in crypt base cells compared to villus tip cells. Ethanol feeding elevated the activities of SOD (76–190%) and catalase (20–150%) in enterocytes all along the crypt-villus axis compared to the controls. H3 thymidine incorporation into DNA of enterocytes was reduced by half in ethanol-fed rats compared to controls.
Conclusions
There is a gradient in the concentration of lipid peroxides in enterocytes across the crypt-villus axis, being high at the villus tip and low at the crypt base in the rat intestine. Ethanol feeding enhanced lipid peroxidation in both villus and crypt cells.
Similar content being viewed by others
References
Persson J, Berg NO, Sjölund K, Stenling R, Magnusson PH. Morphologic changes in the small intestine after chronic alcohol consumption. Scand J Gastroenterol 1990;25:173–184.
Persson J. Alcohol and the small intestine. Scand J Gastroenterol 1991;26:3–15.
Kaur J, Jaswal VM, Nagpaul JP, Mahmood A. Effect of chronic alcohol administration on the absorptive functions of the rat small intestine. Alcohol 1993;10:299–302.
Beck IT, Dinda PK. Acute exposure of small intestine to ethanol: effect on morphology and function. Dig Dis Sci 1981;26:817–838.
Hall PA, Coate PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract. The impotance of apoptosis. J Cell Sci 1994;107:3569–3577.
Potten CS, Epithelial cell growth and differentiation II. Intestinal apoptosis. Am J Physiol (Gastroenterology) 1997;737:G253–G257.
Turan A, Mahmood A. The profile of antioxidant systems and lipid peroxidation across the crypt-villus axis in rat intestine. Dig Dis Sci 2007;52:1840–1844.
Turan A, Gill R, Dudeja PK, Mohan H, Mahmood A. Effect of fat feeding on pro-oxidant and antioxidant enzyme systems in rat intestine: possible role in the turnover of enterocytes. Dig Dig Sci 2009;54:1229–1236.
Salespuro M. Bacterocolonic pathway for ethanol oxidation. Characterization and implications. Ann Med 1996;28:195–200.
Lieber CS. Ethnic and gender differences in ethanol metabolism. Alcohol Clin Exp Res 2000;24:417–418.
Weiser MM. Intestinal epithelial cell surface membrane glycoprotein synthesis. J Biol Chem 1973;248:2536–2541.
Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 1956;62:315–323.
Amador E, Wacker WEC. Enzymatic methods used for diagnosis. In: Methods of Biochemical Analysis, Glick D (ed). New York: Wiley; 1976;Vol 13:265–356.
Dahlqvist A. Method for assay of intestinal disaccharidases. Anal Biochem 1964;7:18–25.
Horn HD, Brun FM. Methods of enzymatic analysis, Bergmeyer HU (ed). Academic Press, New York; 1971: 875–881.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophy 1978;186:189–195.
Luck H. Methods of enzymatic analysis, New York, USA: Academic; 1971:885–888.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol 1978;52:302–310.
Dudeja PK, Brasitus TA. Inactivation of rat small intestinal brush-border membrane alkaline phosphatase by oxygen free radicals. Gastroenterology 1993;105:357–366.
Lowry OH, Rosebrough NJ, Farral AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–275.
Alvarado F, Mahmood A. Cotransport of organic solutes and sodium ions in the small intestine: a General Model. Amino acid transport. Biochemistry 1974;13:2882–2890.
Jackson JH, Schraufstatter IU, Hyslop PA, et al. Role of oxidants in DNA damage. Hydroxyl radical mediates the synergistic DNA damaging effects of asbestos and cigarette smoke. J Clin Invest 1987;80:1090–1095.
Kaur M, Kaur J, Ojha S, Mahmood A. Ethanol effects on lipid peroxidation and glutathione-mediated defense in rat small intestine: role of dietary fats. Alcohol 1998;15:65–69.
Nordmann R, Ribiere C, Rauach H. Ethanol-induced lipid per-oxidation and oxidative stress in extrahepatic tissues. Alcohol Alcohol 1990;25:231–237.
Turini ME, Thomson ABR, Clandinin MT. Lipid composition and peroxide levels of mucosal cells in the rat large intestine in relation to dietary fat. Lipids 1991;26:431–440.
Bhalla S, Mahmood S, Mahmood A. Effect of prenatal exposure to ethanol on postnatal development of intestinal transport functions in rats. Eur J Nutr 2004;43:109–115.
Peres WA, Carmo MG, Zucoloto S, Iglesias AC, Braulio VB. Ethanol intake inhibits growth of the epithelium in the intestine of pregnant rats. Alcohol 2004;33:83–89.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalra, A.K., Gupta, S., Turan, A. et al. Ethanol-induced changes in lipid peroxidation of enterocytes across the crypt-villus axis in rats. Indian J Gastroenterol 29, 23–27 (2010). https://doi.org/10.1007/s12664-010-0003-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-010-0003-6