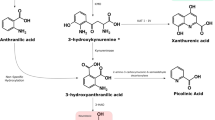
Abstract
Schizophrenia has a clear sexual dimorphism in age of onset and progression. The underlying mechanisms of this dimorphism are not known, but may be found in the interactions of sex hormones with the tryptophan catabolising kynurenine pathway. Schizophrenia is associated with general inflammation and disruption of glutamatergic and dopaminergic signalling. Metabolites of the kynurenine pathway have been shown to be immunomodulatory and have effects on glutamatergic and dopaminergic signalling. This review discusses the currently available literature on sex hormones and their effect on the kynurenine pathway in the context of the glutamatergic, dopaminergic and immunological features of schizophrenia.




Similar content being viewed by others
Abbreviations
- 3HAA:
-
3-hydroxy anthranilic acid
- 3HAO:
-
3-hydroxyanthranilate
- 3HK:
-
3-hydroxy kynurenine
- ACMS:
-
Aminocarboxymuconate semialdehyde
- ACMSD:
-
Aminocarboxymuconate semialdehyde decarboxylase
- IDO1:
-
Indoleamine 2,3-dioxygenase
- IFN-γ:
-
Interferon gamma
- KAT:
-
Kynurenine aminotransferase
- KMO:
-
Kynurenine monooxygenase
- KP:
-
Kynurenine Pathway
- KYN:
-
Kynurenine
- KYNA:
-
Kynurenic acid
- NMDAR:
-
N-methyl-D-aspartate receptor
- PIC:
-
Picolinic acid
- QPRT:
-
Quinolinate phosphoribosyltransferase
- QUIN:
-
Quinolinic acid
- TDO:
-
Tryptophan-2,3-dioxygenase
- TRP:
-
Tryptophan
References
Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M (2000) Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 97:8104–8109. doi:10.1073/pnas.97.14.8104
Baker AE, Brautigam VM, Watters JJ (2004) Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology 145:5021–5032. doi:10.1210/en.2004-0619
Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EMC, Macchiarulo A, Vacca C, Iannitti R, Tissi L, Volpi C, Belladonna ML, Orabona C, Bianchi R, Lanz TV, Platten M, Della Fazia MA, Piobbico D, Zelante T, Funakoshi H, Nakamura T, Gilot D, Denison MS, Guillemin GJ, DuHadaway JB, Prendergast GC, Metz R, Geffard M, Boon L, Pirro M, Iorio A, Veyret B, Romani L, Grohmann U, Fallarino F, Puccetti P (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190. doi:10.1038/nature13323
Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D (2012) Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology 37:1901–1911. doi:10.1016/j.psyneuen.2012.04.001
Braidman IP, Rose DP (1971) Effects of sex hormones on three glucocorticoid-inducible enzymes concerned with amino acid metabolism in rat liver. Endocrinology 89:1250–1255
Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM (2007) Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids 72:381–405. doi:10.1016/j.steroids.2007.02.003
Cepeda C, André V.M, Jocoy E.L, Levine M.S, (2009). NMDA and Dopamine: Diverse mechanisms applied to interacting receptor systems, in: Biology of the NMDA Receptor. pp. 1–13. doi:NBK5280 [bookaccession]
Chauvel V, Vamos E, Pardutz A, Vecsei L, Schoenen J, Multon S (2012) Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. Exp Neurol 236:207–214. doi:10.1016/j.expneurol.2012.05.002
Chen Y, Guillemin GJ (2009) Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2:1–19
Connell BJ, Crosby KM, Richard MJP, Mayne MB, Saleh TM (2007) Estrogen-mediated neuroprotection in the cortex may require NMDA receptor activation. Neuroscience 146:160–169. doi:10.1016/j.neuroscience.2007.01.014
Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, Lakkis FG, Fuchs D, Pober JS, Tellides G (2007) Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol 179(8):5246–5254. http://www.ncbi.nlm.nih.gov/pubmed/17911610. Accessed 22 Dec 2013
Dantzer R (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol 500:399–411. doi:10.1016/j.ejphar.2004.07.040
Dewick PM (2001) Medicinal natural products, 2nd edn. Wiley, Chichester. doi:10.1002/0470846275
Dhandapani KM, Wade FM, Mahesh VB, Brann DW (2005) Astrocyte-derived transforming growth factor-{beta} mediates the neuroprotective effects of 17{beta}-estradiol: involvement of nonclassical genomic signaling pathways. Endocrinology 146(6):2749–2759. doi:10.1210/en.2005-0014
El-Zoghby SM, El-Sewedy SM, Saad AA, Mostafa MH, Ebied SM, Abdel-Tawab GA (1976) In vitro trials to counteract the inhibitory effect of beta-oestradiol and ethynyloestradiol on the B6-dependent kynurenine aminotransferase enzyme. Biochem Pharmacol 25:2411–2413. doi:10.1016/0006-2952(76)90040-X
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313:96–98
Erhardt S, Schwieler L, Engberg G (2003) Kynurenic acid and schizophrenia. Adv Exp Med Biol 527:155–165
Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214. doi:10.1038/mp.2012.110
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR (1991) Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56:2007–2017
Gogos A, Kwek P, Chavez C, van den Buuse M (2010) Estrogen treatment blocks 8-hydroxy-2-dipropylaminotetralin- and apomorphine-induced disruptions of prepulse inhibition: involvement of dopamine D1 or D2 or serotonin 5-HT1A, 5-HT2A, or 5-HT7 receptors. J Pharmacol Exp Ther 333:218–227. doi:10.1124/jpet.109.162123
Gogos A, Kwek P, van den Buuse M (2012) The role of estrogen and testosterone in female rats in behavioral models of relevance to schizophrenia. Psychopharmacology 219:213–224. doi:10.1007/s00213-011-2389-y
Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C (2011) Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 37:504–513. doi:10.1093/schbul/sbr030
Gos T, Myint A-M, Schiltz K, Meyer-Lotz G, Dobrowolny H, Busse S, Müller UJ, Mawrin C, Bernstein HG, Bogerts B, Steiner J (2014) Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain Behav Immun. doi:10.1016/j.bbi.2014.05.012
Grant RS, Coggan SE, Smythe GA (2009) The physiological action of picolinic acid in the human brain. Int J Tryptophan Res. 2:71–79
Guillemin GJ (2012) Quinolinic acid, the inescapable neurotoxin. FEBS J 279:1356–1365. doi:10.1111/j.1742-4658.2012.08485.x
Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ, Croitoru J, Brew BJ (2001) Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 78:842–853
Guillemin GJ, Smythe G, Takikawa O, Brew BJ (2005) Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49:15–23. doi:10.1002/glia.20090
Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21:7463–7473
Holtze M, Saetre P, Erhardt S, Schwieler L, Werge T, Hansen T, Nielsen J, Djurovic S, Melle I, Andreassen OA, Hall H, Terenius L, Agartz I, Engberg G, Jönsson EG, Schalling M (2011) Kynurenine 3-monooxygenase (KMO) polymorphisms in schizophrenia: an association study. Schizophr Res 127:270–272. doi:10.1016/j.schres.2010.10.002
Huang Y, Huang YL, Zhang S, Zhu YC, Yao T (2004) Estradiol acutely attenuates glutamate-induced calcium overload in primarily cultured rat hippocampal neurons through a membrane receptor-dependent mechanism. Brain Res 1026:254–260. doi:10.1016/j.brainres.2004.08.038
Huckle PL, Palia SS (1993) Managing resistant schizophrenia. Br J Hosp Med 50:467–471
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY (2001) 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76:1521–1531
Iqbal N, Asnis GM, Wetzler S, Kay SR, van Praag HM (1991) The role of serotonin in schizophrenia. New findings Schizophr Res. 5:181–182
Kahlfuß S, Simma N, Mankiewicz J, Bose T, Lowinus T, Klein-Hessling S, Sprengel R, Schraven B, Heine M, Bommhardt U (2014) Immunosuppression by N-methyl-D-aspartate receptor antagonists is mediated through inhibition of Kv1.3 and KCa3.1 channels in T cells. Mol Cell Biol 34:820–831. doi:10.1128/MCB.01273-13
Kapoor R, Lim KS, Cheng A, Garrick T, Kapoor V (2006) Preliminary evidence for a link between schizophrenia and NMDA-glycine site receptor ligand metabolic enzymes, d-amino acid oxidase (DAAO) and kynurenine aminotransferase-1 (KAT-1). Brain Res 1106:205–210. doi:10.1016/j.brainres.2006.05.082
Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl M, Sellgren C, Schwieler L, Engberg G, Schuppe-Koistinen I, Erhardt S (2014) Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 7:15–22. doi:10.4137/IJTR.S16800
Kemp JA, Foster AC, Wong EHF (1987) Non-competitive antagonists of excitatory amino acid receptors. Trends Neurosci 10:294–298. doi:10.1016/0166-2236(87)90176-7
Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, Schuett C (2010) Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE 5:1–12. doi:10.1371/journal.pone.0011825
Knox WE (1951) Two mechanisms which increase in vivo the liver tryptophan peroxidase activity: specific enzyme adaptation and stimulation of the pituitary adrenal system. Br J Exp Pathol. 32:462–469
Kulkarni J, Riedel A, De Castella A (2001) Estrogen—a potential treatment for schizophrenia. Schizophr res. 48:137–144
Lekiem JE, Brown RR, Rose DP (1975) Vitamin B6 requirements of women using oral contraceptives. The Am j of clinic nutr. 28:535–541
Liu L, Wang Z (2013) Estrogen attenuates lipopolysaccharide-induced nitric oxide production in macrophages partially via the nongenomic pathway. Cell Immunol 286:53–58. doi:10.1016/j.cellimm.2013.11.004
Locklear MN, Cohen AB, Jone a, Kritzer MF (2014) Sex differences distinguish intracortical glutamate receptor-mediated regulation of extracellular dopamine levels in the prefrontal cortex of adult rats. Cortex, Cereb. doi:10.1093/cercor/bhu222
Martin LF, Freedman R (2007) Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol 78:225–246. doi:10.1016/S0074-7742(06)78008-4
McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, Krause V, Kumar RA, Grozeva D, Malhotra D, Walsh T, Zackai EH, Kaplan P, Ganesh J, Krantz ID, Spinner NB, Roccanova P, Bhandari A, Pavon K, Lakshmi B, Leotta A, Kendall J, Lee Y-H, Vacic V, Gary S, Iakoucheva LM, Crow TJ, Christian SL, Lieberman JA, Stroup TS, Lehtimäki T, Puura K, Haldeman-Englert C, Pearl J, Goodell M, Willour VL, Derosse P, Steele J, Kassem L, Wolff J, Chitkara N, McMahon FJ, Malhotra AK, Potash JB, Schulze TG, Nöthen MM, Cichon S, Rietschel M, Leibenluft E, Lajonchere CM, Sutcliffe JS, Skuse D, Gill M, Gallagher L, Mendell NR, Craddock N, Owen MJ, O’Donovan MC, Shaikh TH, Susser E, Delisi LE, Sullivan PF, Deutsch CK, Rapoport J, Levy DL, King M-C, Sebat J (2009) Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet 41:1223–1227. doi:10.1038/ng.474
Moursi GE, Abdel-Daim MH, Kelada NL, Abdel-Tawab GA, Girgis LH (1970) The influence of sex, age, synthetic oestrogens, progestogens and oral contraceptives on the excretion of urinary tryptophan metabolites. Bull World Health Organ 43:651–661
Müller N, Myint A-M, Schwarz MJ, Muller N, Myint AMJ, Schwarz M (2011) Kynurenine pathway in schizophrenia: pathophysiological and therapeutic aspects. Curr Pharm Des 17:130–136. doi:10.2174/138161211795049552
Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpé S, Steinbusch HWM, Leonard BE, Kim YK (2011) Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav Immun 25:1576–1581. doi:10.1016/j.bbi.2011.05.005
Nilsen J, Chen S, Brinton RD (2002) Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res 930:216–234
Notarangelo FM, Wilson EH, Horning KJ, Wilson EH, Thomas MaR, Harris TH, Fang Q, Hunter CA, Schwarcz R (2013). Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: Implications for schizophrenia. Schizophr Res. doi:10.1016/j.schres.2013.11.011
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203. doi:10.1038/nature10491
Ormstad H, Verkerk R, Aass HCD, Amthor KF, Sandvik L (2013) Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J Mol Neurosci. doi:10.1007/s12031-013-0097-2
Pemberton LA, Kerr SJ, Smythe G, Brew BJ (1997) Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res 17:589–595
Picchioni MM, Murray RM (2007) Schizophr BMJ 335:91–95. doi:10.1136/bmj.39227.616447.BE
Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, Ell PJ (2006) First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry 11:118–119. doi:10.1038/sj.mp.4001751
Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM (2003) Para/autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus 13:226–234. doi:10.1002/hipo.10075
Prendergast G, Metz R, Muller AJ, Merlo LMF, Mandik-nayak L (2014) IDO2 in immunomodulation and autoimmunity. Immunol, Front. doi:10.3389/fimmu.2014.00585
Pulver AE, Brown CH, Wolyniec P, McGrath J, Tam D, Adler L, Carpenter WT, Childs B (1990) Schizophrenia: age at onset, gender and familial risk. Acta Psychiatr Scand 82:344–351
Riecher-Rössler A, Häfner H (2000) Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl 102:58–62
Riecher-Rössler A, Häfner H, Stumbaum M, Maurer K, Schmidt R (1994) Can estradiol modulate schizophrenic symptomatology? Schizophr Bull 20:203–214
Rose DP (1972) Aspects of tryptophan metabolism in health and disease: a review. J Clin Pathol 25:17–25
Salter M, Pogson CI (1985) The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J 229:499–504
Sandberg A, Rosethal H, Slaunwhite W Jr (1969) Certain metabolic effects of estrogens. Metabolic effects of gonadal hormones and contraceptive steroids. Plenum Press, New York, pp 367–378
Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R (2011) Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 37:1147–1156. doi:10.1093/schbul/sbq112
Schröcksnadel K, Widner B, Bergant A, Neurauter G, Schennach H, Schröcksnadel H, Fuchs D (2003) Longitudinal study of tryptophan degradation during and after pregnancy. Life Sci 72:785–793
Stevens JR (1982) The neuropathology of schizophrenia. Psychol Med 12:695–700
van OS J, Kapur S (2009) Schizophr Lancet 374:635–645. doi:10.1016/S0140-6736(09)60995-8
Vécsei L, Miller J, MacGarvey U, Flint Beal M (1992) Kynurenine and probenecid inhibit pentylenetetrazol- and NMDLA-induced seizures and increase kynurenic acid concentrations in the brain. Brain Res Bull 28:233–238. doi:10.1016/0361-9230(92)90184-Y
Weaver CE, Park-Chung M, Gibbs TT, Farb DH (1997) 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res 761:338–341
Wolf H, Walter S, Brown RR, Arend RA (1980) Effect of natural oestrogens on tryptophan metabolism: evidence for interference of oestrogens with kynureninase. Scand J Clin Lab Invest 40:15–22
Acknowledgments
The authors would like to thank M. James Hutson for his assistance with Fig. 4. Ms Josien de Bie is supported by an international scholarship from Macquarie University. Prof Guillemin is supported by the National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC). Prof Guillemin is also a recipient of the Australian Research Council Future Fellowship Award (FT120100397). Dr Lim is a recipient of the Society of Mental Health Research (SMHR) Australia Early Career Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Bie, J., Lim, C.K. & Guillemin, G.J. Kynurenines, Gender and Neuroinflammation; Showcase Schizophrenia. Neurotox Res 30, 285–294 (2016). https://doi.org/10.1007/s12640-016-9641-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-016-9641-5