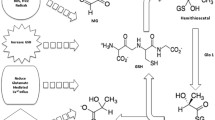
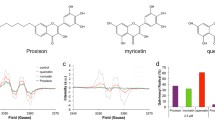
Abstract
Few studies have been undertaken on the relationship of the structure of flavones and neuroprotection. Previously, we described the structural determinants of the neuroprotective activity of some natural flavones in cerebellar granule neurons in culture against an oxidative insult (H2O2). In the present work, we analyzed anti-oxidant activity, cellular iron, and Ca2+ levels and cellular bioavailability of neuroprotective and nonneuroprotective flavones in the same experimental paradigm. Oxidative cellular damage produced by H2O2 was prevented by all of the studied flavones with rather similar potency for all of them. Labile Iron Pool was neither affected by protective nor nonprotective flavones. Intracellular Ca2+ homeostasis was not affected by protective flavones either. Nonetheless, fisetin, the nonprotective flavone, decreased Ca2+ levels modifying Ca2+ homeostasis. Methylation of the catechol group, although weakens anti-oxidant capacity, keeps the neuroprotective capacity with less degradation and lower toxicity, constituting promising structural alternatives as leads for the design of neuroprotective molecules.









Similar content being viewed by others
Notes
Because they all share the basic backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran-4-one), the molecules studied can be primarily considered as flavones and as such are taken in this work, knowing that as hydroxyl flavones they can also be considered as flavonols.
References
Almajano MP, Vila I, Gines S (2011) Neuroprotective effects of white tea against oxidative stress-induced toxicity in striatal cells. Neurotox Res 20:372–378
Andersen OM, Markham KR (2010) Flavonoids: chemistry, biochemistry and applications. CRC Press, Boca Raton
Arredondo F, Echeverry C, Abin-Carriquiry JA, Blasina F, Antúnez K, Jones DP, Go YM, Liang YL, Dajas F (2010) After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic Biol Med 49:738–747
Bors W, Heller W, Michel C, Saran M (1990) Radical chemistry of flavonoid antioxidants. Adv Exp Med Biol 264:165–170
Breuer W, Epsztejn S, Millgram P, Cabantchik ZI (1995) Transport of iron and other transition metals into cells as revealed by a fluorescent probe. Am J Physiol 268:1354–1361
Chan EC, Pannangpetch P, Woodman OL (2000) Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure–activity relationships. Cardiovasc Pharmacol 35:326–333
Chang H, Mi M, Ling W, Zhu J, Zhang Q, Wei N, Zhou Y, Tang Y, Yuan J (2008) Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch Pharm Res 31:1137–1144
Clifford M (2001) A nomenclature for phenols with special reference to tea. Crit Rev Food Sci Nutr 41:393–397
Dajas F (2012) Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol 143:383–396
Dajas F, Rivera F, Blasina F, Arredondo F, Echeverry C, Lafon L, Morquio A, Heizen H (2003) Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res 5:425–432
Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 89:271–277
Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M (1993) A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med 15:353–363
Echeverry C, Arredondo F, Abin-Carriquiry JA, Midiwo JO, Ochieng C, Kerubo L, Dajas F (2010) Pretreatment with natural flavones and neuronal cell survival after oxidative stress: a structure–activity relationship study. J Agric Food Chem 58:2111–2115
Franklin JL, Johnson EM (1992) Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci 15:501–508
García O, Massieu L (2001) Strategies for neuroprotection against l-trans-2,4-pyrrolidine dicarboxylate-induced neuronal damage during energy impairment in vitro. J Neurosci Res 64:418–428
Harborne JB (ed) (1994) The flavonoids, advances in research since 1986. Chapman and Hall, London
Heijnen CG, Haenen GR, Vekemans JA, Bast A (2001) Peroxynitrite scavenging of flavonoids: structure activity relationship. Environ Toxicol Pharmacol 10:199–206
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem 13:572–584
Juma BF, Yenese A, Midiwo JO, Waterman PG (2001) Flavones and phenylpropenoids in the surface exudate of Psiadia punctulata. Phytochemistry 57:571–574
Kruszewski M (2003) Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res 531:81–92
Li S, Pu XP (2011) Neuroprotective effect of kaempferol against a 1- methyl-4-phenyl-1.2.3.6-tetrahydropyridine-induced mouse model of Parkinson´s disease. Biol Pharm Bull 34:1291–1296
Liu WB, Wang YP (2001) Magnesium lithospermate B inhibits hypoxia-induced calcium influx and nitric oxide release in endothelial cell. Acta Pharmacol Sin 22:1135–1142
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Miller NJ, Rice-Evans C, Davies MJ, Gapinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in prematures neonates. Clin Sci 84:407–412
Mira L, Fernández MT, Santos M, Rocha R, Florencio MH, Jennings KR (2002) Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 36:1199–1208
Mueller H, Kassack MU, Wiese M (2004) Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J Biomol Screen 9:506–515
Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA (2001) Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74:418–425
Okimotoa Y, Watanabea A, Nikia E, Yamashitab T, Noguchia N (2000) A novel fluorescent probe diphenyl-1-pyrenylphosphine to follow lipid peroxidation in cell membranes. FEBS Lett 474:137–140
Ossola B, Kääriäinen TM, Männistö PT (2009) The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf 8:397–409
Peng W, Kuo SM (2003) Flavonoid structure affects the inhibition of lipid peroxidation in Caco-2 intestinal cells at physiological concentrations. J Nutr 133:2184–2187
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Rice-Evans C (2001) Flavonoid antioxidants. Curr Med Chem 8:797–807
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY (2005) Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol 31:135–147
Spencer JP (2009) Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr 4:243–250
Walle T (2009) Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int J Mol Sci 10:5002–5019
Wang H, Joseph JA (1999) Structure–activity relationships of quercetin in antagonizing hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med 27:683–694
Williams RJ, Spencer JPE, Rice-Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med 36:838–849
Yenesew A, Irungu B, Derese S, Midiwo JO, Heydenreich M, Peter MG (2003) Two prenylated flavonoids from the stem bark of Erythrina burttii. Phytochemistry 63:445–448
Acknowledgments
This work was supported by Grant L/ICA/139636 from the International Cooperation and Assistance Office of the OPCW (Organization for the Prohibition of Chemical Weapons), The Hague, The Netherlands. We thank Prof. Prem Ponka for the generous gift of the iron chelator SIH.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Echeverry, C., Arredondo, F., Martínez, M. et al. Antioxidant Activity, Cellular Bioavailability, and Iron and Calcium Management of Neuroprotective and Nonneuroprotective Flavones. Neurotox Res 27, 31–42 (2015). https://doi.org/10.1007/s12640-014-9483-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-014-9483-y