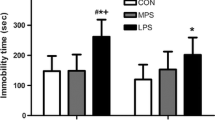
Abstract
Exposure to a variety of stressful events during the last week of pregnancy in rats interferes with the correct progeny development, which in turn leads to delays in motor development, impaired adaptation to stressful conditions, altered sexual behaviour, learning deficits, neuronal development and brain morphology. Many of these alterations have been attributed to changes in dopamine (DA) neurotransmission and occur primarily in the mesolimbic system. We found that prenatally stressed offspring showed higher levels of cells expressing tyrosine hydroxylase (TH) in the ventral tegmental area (VTA) and that these cells were more susceptible to a neurochemical insult with 6-hydroxy-DA (6-OHDA) in adulthood. Moreover, prenatally stressed rats presented differences in terms of the number and asymmetry of neuronal nitric oxide synthase-expressing cells in the VTA and nucleus accumbens, respectively. Similar to the results described for TH-expressing cells, the nitrergic systems were differentially regulated after 6-OHDA lesion in control and prenatally stressed rats. These results indicated that prenatal stress affects the dopaminergic and nitrergic systems in the mesolimbic pathway. In addition, we propose that the mesolimbic areas are more susceptible than the motor areas to a neurochemical insult during adult life.





Similar content being viewed by others
References
Adrover E, Berger MA, Perez AA, Tarazi FI, Antonelli MC (2007) Effects of prenatal stress on dopamine D2 receptor asymmetry in rat brain. Synapse 61(6):459–462
Baier CJ, Katunar MR, Adrover E, Pallares ME, Antonelli MC (2012) Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res 22(1):16–32. doi:10.1007/s12640-011-9305-4
Balda MA, Anderson KL, Itzhak Y (2009) The neuronal nitric oxide synthase (nNOS) gene contributes to the regulation of tyrosine hydroxylase (TH) by cocaine. Neurosci Lett 457(3):120–124. doi:10.1016/j.neulet.2009.04.011
Barros VG, Berger MA, Martijena ID, Sarchi MI, Perez AA, Molina VA, Tarazi FI, Antonelli MC (2004) Early adoption modifies the effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. J Neurosci Res 76(4):488–496
Barthwal MK, Srivastava N, Dikshit M (2001) Role of nitric oxide in a progressive neurodegeneration model of Parkinson’s disease in the rat. Redox Rep Commun Free Radic Res 6(5):297–302
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC (2002) Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res 27(11):1525–1533
Bredt DS, Snyder SH (1992) Nitric oxide, a novel neuronal messenger. Neuron 8(1):3–11
Darnaudery M, Maccari S (2008) Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev 57(2):571–585. doi:10.1016/j.brainresrev.2007.11.004
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88(14):6368–6371
Debeir T, Ginestet L, Francois C, Laurens S, Martel JC, Chopin P, Marien M, Colpaert F, Raisman-Vozari R (2005) Effect of intrastriatal 6-OHDA lesion on dopaminergic innervation of the rat cortex and globus pallidus. Exp Neurol 193(2):444–454. doi:10.1016/j.expneurol.2005.01.007
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175(2):303–317. doi:10.1006/exnr.2002.7891
Estevez AG, Spear N, Thompson JA, Cornwell TL, Radi R, Barbeito L, Beckman JS (1998) Nitric oxide-dependent production of cGMP supports the survival of rat embryonic motor neurons cultured with brain-derived neurotrophic factor. J Neurosci 18(10):3708–3714
Eve DJ, Nisbet AP, Kingsbury AE, Hewson EL, Daniel SE, Lees AJ, Marsden CD, Foster OJ (1998) Basal ganglia neuronal nitric oxide synthase mRNA expression in Parkinson’s disease. Brain Res Mol Brain Res 63(1):62–71
Fride E, Weinstock M (1989) Alterations in behavioral and striatal dopamine asymmetries induced by prenatal stress. Pharmacol Biochem Behav 32(2):425–430
Gomes MZ, Del Bel EA (2003) Effects of electrolytic and 6-hydroxydopamine lesions of rat nigrostriatal pathway on nitric oxide synthase and nicotinamide adenine dinucleotide phosphate diaphorase. Brain Res Bull 62(2):107–115
Gomes MZ, Raisman-Vozari R, Del Bel EA (2008) A nitric oxide synthase inhibitor decreases 6-hydroxydopamine effects on tyrosine hydroxylase and neuronal nitric oxide synthase in the rat nigrostriatal pathway. Brain Res 1203:160–169. doi:10.1016/j.brainres.2008.01.088
Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD, Yoo YM, Kim PK, Chung HT, Billiar TR, Kim YM (2003) Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J Off Publ Fed Am Soc Exp Biol 17(9):1036–1047. doi:10.1096/fj.02-0738com
Hausknecht K, Haj-Dahmane S, Shen RY (2013) Prenatal stress exposure increases the excitation of dopamine neurons in the ventral tegmental area and alters their responses to psychostimulants. Neuropsychopharmacology 38(2):293–301. doi:10.1038/npp.2012.168
Hoque KE, West AR (2012) Dopaminergic modulation of nitric oxide synthase activity in subregions of the rat nucleus accumbens. Synapse 66(3):220–231. doi:10.1002/syn.21503
Huizink AC, Mulder EJ, Buitelaar JK (2004) Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull 130(1):115–142. doi:10.1037/0033-2909.130.1.115
Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Investig 111(2):163–169. doi:10.1172/JCI17638
Katunar M, Saez T, Brusco A, Antonelli M (2009) Immunocytochemical expression of dopamine-related transcription factors Pitx3 and Nurr1 in prenatally stressed adult rats. J Neurosci Res 87:1014–1022
Katunar M, Saez T, Brusco A, Antonelli M (2010) Ontogenetic expression of dopamine-related transcription factors and tyrosine hydroxylase in prenatally stressed rats. Neurotox Res 18(1):69–81
Kim YM, Chung HT, Kim SS, Han JA, Yoo YM, Kim KM, Lee GH, Yun HY, Green A, Li J, Simmons RL, Billiar TR (1999) Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling. J Neurosci 19(16):6740–6747
Kiss JP, Hennings EC, Zsilla G, Vizi ES (1999) A possible role of nitric oxide in the regulation of dopamine transporter function in the striatum. Neurochem Int 34(4):345–350
Klejbor I, Domaradzka-Pytel B, Ludkiewicz B, Wojcik S, Morys J (2004) The relationships between neurons containing dopamine and nitric oxide synthase in the ventral tegmental area. Folia Histochem Cytobiol 42(2):83–87
Kofman O (2002) The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 26(4):457–470
Kuter K, Kolasiewicz W, Golembiowska K, Dziubina A, Schulze G, Berghauzen K, Wardas J, Ossowska K (2011) Partial lesion of the dopaminergic innervation of the ventral striatum induces “depressive-like” behavior of rats. Pharmacol Rep 63(6):1383–1392
Lee C, Park GH, Jang JH (2011) Cellular antioxidant adaptive survival response to 6-hydroxydopamine-induced nitrosative cell death in C6 glioma cells. Toxicology 283(2–3):118–128. doi:10.1016/j.tox.2011.03.004
Lemaire V, Koehl M, Le Moal M, Abrous DN (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA 97(20):11032–11037
Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M (1995) Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci 15(1 Pt 1):110–116
Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI (2010) Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci 4:173. doi:10.3389/fnbeh.2010.00173
McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE (2005) Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol 17(8):475–482. doi:10.1111/j.1365-2826.2005.01331.x
McArthur S, McHale E, Gillies GE (2007) The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex- region- and time-specific manner. Neuropsychopharmacology 32(7):1462–1476. doi:10.1038/sj.npp.1301277
Ohno M, Arai I, Watanabe S (1995) N-Methyl-d-aspartate stimulates dopamine release through nitric oxide formation in the nucleus accumbens of rats. Brain Res 699(2):332–335
Pallares ME, Baier CJ, Adrover E, Monteleone MC, Brocco MA, Antonelli MC (2013) Age-dependent effects of prenatal stress on the corticolimbic dopaminergic system development in the rat male offspring. Neurochem Res 38(11):2323–2335. doi:10.1007/s11064-013-1143-8
Pardon MC, Rattray I (2008) What do we know about the long-term consequences of stress on ageing and the progression of age-related neurodegenerative disorders? Neurosci Biobehav Rev 32(6):1103–1120. doi:10.1016/j.neubiorev.2008.03.005
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM (1995) Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67(3):631–647
Rice F, Jones I, Thapar A (2007) The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand 115(3):171–183. doi:10.1111/j.1600-0447.2006.00895.x
Simola N, Morelli M, Carta AR (2007) The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res 11(3–4):151–167
Singh S, Kumar S, Dikshit M (2010) Involvement of the mitochondrial apoptotic pathway and nitric oxide synthase in dopaminergic neuronal death induced by 6-hydroxydopamine and lipopolysaccharide. Redox Rep Commun Free Radic Res 15(3):115–122. doi:10.1179/174329210X12650506623447
Smidt MP, Burbach JP (2007) How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci 8(1):21–32
Steiner H, Kitai ST (2001) Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. Eur J Neurosci 14(8):1390–1404
Sun H, Guan L, Zhu Z, Li H (2013) Reduced levels of NR1 and NR2A with depression-like behavior in different brain regions in prenatally stressed juvenile offspring. PLoS ONE 8(11):e81775. doi:10.1371/journal.pone.0081775
Ward IL, Weisz J (1984) Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 114(5):1635–1644
Weinstock M (2001) Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol 65(5):427–451
Weinstock M (2008) The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32(6):1073–1086. doi:10.1016/j.neubiorev.2008.03.002
Yuste JE, Echeverry MB, Ros-Bernal F, Gomez A, Ros CM, Campuzano CM, Fernandez-Villalba E, Herrero MT (2012) 7-Nitroindazole down-regulates dopamine/DARPP-32 signaling in neostriatal neurons in a rat model of Parkinson’s disease. Neuropharmacology 63(7):1258–1267. doi:10.1016/j.neuropharm.2012.07.031
Zhang J, Dawson VL, Dawson TM, Snyder SH (1994) Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science 263(5147):687–689
Acknowledgments
The authors wish to thank Dr. Ana Maria Adamo and Ms. María Florencia Almeira Gubiani (Instituto de Química y Fisicoquímica Biológicas, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina) for their valuable assistance in the stereotaxical experiments as well as Mr. Majid Amar (INSERM UMRS 975, CRICM, ICM, Thérapeutique Expérimentale de la Neurodégénérescence, Paris, France) for his assistance in IHC experiments. We are also indebted to Martin Brahamian and Mercedes Imsem (Universidad de Buenos Aires, Buenos Aires, Argentina) for their help and supervision in the management and care of the rats. The skilful technical assistance of Mrs. Susana Buglione (Instituto de Química y Fisicoquímica Biológicas, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina) is greatly appreciated. This research was supported by Grants from CONICET (PIP 2065), ANPCYT (PICT 31981 and PICT 0040), and Bernardo Houssay Program (CONICET/MINCyT/France Embassy).
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baier, C.J., Pallarés, M.E., Adrover, E. et al. Intrastriatal 6-OHDA Lesion Differentially Affects Dopaminergic Neurons in the Ventral Tegmental Area of Prenatally Stressed Rats. Neurotox Res 26, 274–284 (2014). https://doi.org/10.1007/s12640-014-9479-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-014-9479-7