Abstract
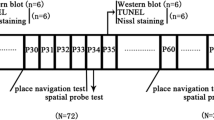
Propofol and sevoflurane are commonly used drugs in pediatric anesthesia. Exposure of newborn rats to a variety of anesthetics has been shown to induce apoptotic neurodegeneration in the developing brain. Newborn Wistar rats were treated with repeated intraperitoneal injections of propofol or sevoflurane inhalation and compared to controls. Brains were examined histopathologically using the De Olmos cupric silver staining. Additionally, a summation score of the density of apoptotic cells was calculated for every brain. Spatial memory learning was assessed by the Morris Water Maze (MWM) test and the hole board test, performed in 7 weeks old animals who underwent the same anesthetic procedure. Brains of propofol-treated animals showed a significant higher neurodegenerative summation score (24,345) when compared to controls (15,872) and to sevoflurane-treated animals (18,870). Treated animals also demonstrated persistent learning deficits in the hole board test, whereas the MWM test revealed no differences between both groups. Among other substances acting via GABAA agonism and/or NMDA antagonism propofol induced neurodegeneration in newborn rat brains whereas a sevoflurane based anesthesia did not. The significance of these results for clinical anesthesia has not been completely elucidated. Future studies have to focus on the detection of safe anesthetic strategies for the developing brain.

Similar content being viewed by others
References
Anand KJ, Soriano SG (2004) Anesthetic agents and the immature brain: are these toxic or therapeutic? Anesthesiology 101:527–530
Bert B, Fink H, Huston JP, Voits M (2002) Fischer 344 and Wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiol Learn Mem 78:11–22
Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C (2002) Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA 99:15089–15094
Bittigau P, Sifringer M, Ikonomidou C (2003) Antiepileptic drugs and apoptosis in the developing brain. Ann NY Acad Sci 993:103–114; discussion 123–104
Cattano D, Young C, Straiko MM, Olney JW (2008) Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg 106:1712–1714
De Olmos JS, Ingram WR (1971) An improved cupric-silver method for impregnation of axonal and terminal degeneration. Brain Res 33:523–529
Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB (1990) Phenobarbital for febrile seizures—effects on intelligence and on seizure recurrence. N Engl J Med 322:364–369
Fredriksson A, Ponten E, Gordh T, Eriksson P (2007) Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 107:427–436
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A et al (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394
Hapfelmeier G, Schneck H, Kochs E (2001) Sevoflurane potentiates and blocks GABA-induced currents through recombinant alpha1beta2gamma2 GABAA receptors: implications for an enhanced GABAergic transmission. Eur J Anaesthesiol 18:377–383
Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW (2001) Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol 62:401–405
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Johnson SA, Young C, Olney JW (2008) Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol 20:21–28
Kay B, Rolly G (1977) I.C.I. 35868, a new intravenous induction agent. Acta Anaesthesiol Belg 28:303–316
Laegreid L, Hagberg G, Lundberg A (1992) Neurodevelopment in late infancy after prenatal exposure to benzodiazepines—a prospective study. Neuropediatrics 23:60–67
Lanigan C, Sury M, Bingham R, Howard R, Mackersie A (1992) Neurological sequelae in children after prolonged propofol infusion. Anaesthesia 47:810–811
Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H (2007) Effects of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology 53:942–950
Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, Kalayjian LA, Kanner A, Liporace JD, Pennell PB, Privitera M, Loring DW (2009) Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 360:1597–1605
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Motsch J, Roggenbach J (2004) Propofol infusion syndrome. Anaesthesist 53:1009–1022; quiz 1023–1004
Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, Almirall D, Wessel DL, Jonas RA, Wernovsky G (2003) Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr 143:67–73
Nikizad H, Yon JH, Carter LB, Jevtovic-Todorovic V (2007) Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann NY Acad Sci 1122:69–82
Olney JW, Young C, Wozniak DF, Ikonomidou C, Jevtovic-Todorovic V (2004) Anesthesia-induced developmental neuroapoptosis. Does it happen in humans? Anesthesiology 101:273–275
Parke TJ, Stevens JE, Rice AS, Greenaway CL, Bray RJ, Smith PJ, Waldmann CS, Verghese C (1992) Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ 305:613–616
Soriano SG, Anand KJ, Rovnaghi CR, Hickey PR (2005) Of mice and men: should we extrapolate rodent experimental data to the care of human neonates? Anesthesiology 102:866–868; author reply 868–869
Trotter C, Serpell MG (1992) Neurological sequelae in children after prolonged propofol infusion. Anaesthesia 47:340–342
Voits M, Fink H, Gerhardt P, Huston JP (1995) Application of ‘nose-poke habituation’ validation with post-trial diazepam- and cholecystokinin-induced hypo- and hypermnesia. J Neurosci Methods 57:101–105
Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG (2008) The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology 108:251–260
Wise-Faberowski L, Raizada MK, Sumners C (2001) Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth Analg 93:1281–1287
Zhang G, Dong Y, Zhang B, Ichinose F, Wu X, Culley DJ, Crosby G, Tanzi RE, Xie Z (2008) Isoflurane-induced caspase-3 activation is dependent on cytosolic calcium and can be attenuated by memantine. J Neurosci 28:4551–4560
Acknowledgments
This study was funded by institutional resources of the Department of Anesthesiology and Intensive Care Medicine, and of the Children’s Hospital, Campus Virchow Klinikum, Charité-Universitaetsmedizin in Berlin, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Bercker and B. Bert contributed equally to this article.
Rights and permissions
About this article
Cite this article
Bercker, S., Bert, B., Bittigau, P. et al. Neurodegeneration in Newborn Rats Following Propofol and Sevoflurane Anesthesia. Neurotox Res 16, 140–147 (2009). https://doi.org/10.1007/s12640-009-9063-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9063-8