Abstract
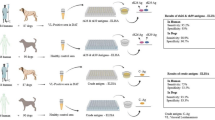
Although canids are regarded as major reservoir hosts for Leishmania infantum, feline leishmaniasis are reported sporadically from different endemic foci of Mediterranean visceral leishmaniasis (VL). Despite the risk of parasite transmission between human and other animals, most of the studies are limited to dogs and few studies are focused to investigate Leishmania sp. among other mammals. This project was aimed to detect L. infantum antibodies of cats in two VL endemic regions of Iran by Fucose Mannose Ligand (FML) and soluble L. infantum antigen (SLA) ELISA. Forty nine stray cats of different age and sex, from Fars and Ardabil provinces (two VL endemic loci of Iran) were sampled, then tested for L. infantum by FML and SLA-ELISA. Sixteen percent (8/49) of cat sera were reported positive by FML-ELISA. SLA-ELISA showed 18.3% (9/48) positive cases in cats. Sensitivity of FML-ELISA was calculated 57% and SLA ELISA 25%. Specificity of FML and SLA ELISA were assessed 78% and 68% respectively. Kappa coefficient of agreement between FML and SLA-ELISA was detected on 0.45. As feline leishmaniasis could be a potential risk in endemic areas, FML-ELISA could be considered as an appropriate examination to detect leishmaniasis in cats.



Similar content being viewed by others
References
Alborzi A, Pouladfar GR, Fakhar M, Motazedian MH, Hatam GR, Kadivar MR (2008) Isolation of Leishmania tropica from a patient with visceral leishmaniasis and disseminated cutaneous leishmaniasis, southern Iran. Am J Trop Med Hyg 79(3):435–437
Alves-Martin MF, dos Santos M, Paixão DT, da Silva M, da Silva Tenório ML, Alves WA, Starke-Buzetti VBR, Pereira Lucheis SB (2017) Detection of Leishmania spp. using parasitological, serological and molecular assays in asymptomatic and sick cats from an endemic area of visceral leishmaniosis in Brazil. Asian Pac. J. Trop. 7(11):659–664. https://doi.org/10.12980/apjtd.12987.12017D12987-12100
Benassi JC, Benvenga GU, Ferreira HL, Pereira VF, Keid LB, Soares R, de Sousa Oliveira TMF (2017) Detection of Leishmania infantum DNA in conjunctival swabs of cats by quantitative real-time PCR. Exp Parasitol 177:93–97. https://doi.org/10.1016/j.exppara.2017.1004.1004
Cabrera G, Da Silva VO, Da Costa RT, Reis AB, Mayrink W, Genaro O, Palatnik-de-Sousa CB (1999) The fucose-mannose ligand-ELISA in the diagnosis and prognosis of canine visceral leishmaniasis in Brazil. Am J Trop Med Hyg 61(2):296–301. https://doi.org/10.4269/ajtmh.1999.4261.4296
Coura FM, Passos SKP, Pelegrino MDOF, Leme FDOP, Paz GF, Gontijo CMF, Costa-Val APD (2018) Serological, molecular, and microscopic detection of Leishmania in cats (Felis catus) in Belo Horizonte, Minas Gerais State. Brazil. Rev Bras Parasitol Vet 27(4):570–574. https://doi.org/10.1590/S1984-296120180052
Dedola C, Zobba R, Varcasia A, Visco S, Alberti A, Pipia A, Scala A, Parpaglia MP (2018) Serological and molecular detection of Leishmania infantum in cats of Northern Sardinia, Italy. Vet Parasitol Reg Stud Rep 13:120–123. https://doi.org/10.1016/j.vprsr.2018.1005.1003
Diakou A, Papadopoulos E, Lazarides K (2009) Specific anti-Leishmania spp. antibodies in stray cats in Greece. J Feline Med Surg 11(8): 728–730. https://doi.org/10.1016/j.jfms.2008.1001.1009
Edrissian G, Nadim A, Ardehali S (1999) Visceral leishmaniasis: the Iranian experiences. Arch Iran Med 1(1):22–26
Edrissian GH, Darabian P (1979) A comparison of enzyme-linked immunosorbent assay and indirect fluorescent antibody test in the sero-diagnosis of cutaneous and visceral leishmaniasis in Iran. Trans R Soc Trop Med Hyg 73(3):289–292. https://doi.org/10.1016/0035-9203(1079)90084-90081
Ghatei MA, Hatam G, Hossini M, Sarkari B (2009) Performance of latex agglutination test (KAtex) in diagnosis of visceral leishmaniasis in Iran. Iran J Immunol 6(4):202–207
Hatam GR, Adnani SJ, Asgari Q, Fallah E, Motazedian MH, Sadjjadi SM, Sarkari B (2010) First report of natural infection in cats with Leishmania infantum in Iran. Vector Borne Zoonotic Dis 10(3):313–316. https://doi.org/10.1089/vbz.2009.0023
Herwaldt B (1999) Leishmaniasis. Lancet 354:1191–1199
Kumar R, Pai K, Pathak K, Sundar S (2001) Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin Diagn Lab Immunol 8(6):1220–1224. https://doi.org/10.1128/CDLI.1228.1226.1220-1224.2001
Lima VMF, Biazzono DL, Silva AC, Correa APFL, Luvizotto MCR (2005) Serological diagnosis of visceral leishmaniasis by an enzyme immunoassay using protein A in naturally infected dogs. Pesq Vet Bras 25(4): 215–218. https://doi.org/10.1590/S0100-1736X2005000400005
Longoni SS, López-Cespedes A, Sánchez-Moreno M, Bolio-Gonzalez ME, Sauri-Arceo CH, Rodríguez-Vivas RI, Marín C (2012) Detection of different Leishmania spp. and Trypanosoma cruzi antibodies in cats from the Yucatan Peninsula (Mexico) using an iron superoxide dismutase excreted as antigen. Comp Immunol Microbiol Infect Dis 35(5): 469–476. https://doi.org/10.1016/j.cimid.2012.1004.1003
Maia C, Nunes M, Campino L (2008) Importance of cats in zoonotic leishmaniasis in Portugal. Vector Borne Zoonotic Dis 8(4):555–560
Martín-Sánchez J, Acedo C, Muñoz-Pérez M, Pesson B, Marchal O, Morillas-Márquez F (2007) Infection by Leishmania infantum in cats: epidemiological study in Spain. Vet Parasitol 145(3):267–273. https://doi.org/10.1016/j.vetpar.2006.1011.1005
Melby P, Neva F, Sacks D (1989) Profile of human T cell response to leishmanial antigens. Analysis by immunoblotting. J Clin Invest 83(6):1868–1675. https://doi.org/10.1172/JCI114093
Metzdorf IP, Junior MSDCL, Matos DM, de Souza Filho FC, de Souza Tsujisaki AF, Franco RA, Shapiro KG, de Almeida Borges F (2017) Molecular characterization of Leishmania infantum in domestic cats in a region of Brazil endemic for human and canine visceral leishmaniasis. Acta Trop 166:121–125. https://doi.org/10.1016/j.actatropica.2016.1011.1013
Mikaeili F, Fakhar M, Sarkari B, Motazedian MH, Hatam G (2007) Comparison of serological methods (ELISA, DAT and IFA) for diagnosis of visceral leishmaniasis utilizing an endemic strain. Iran J Immunol 4(2): 116–121
Mohammadi-Ghalehbin B, Hatam GR, Sarkari B, Mohebali M, Zarei Z, Jaberipour M, Bohlouli S (2011) A Leishmania infantum FML-ELISA for the detection of symptomatic and asymptomatic canine visceral leishmaniasis in an endemic area of Iran. Iran J Immunol 8(4):244–250
Mohebali M, Edrissian GH, Shirzadi MR, Akhoundi B, Hajjaran H, Zarei Z, Molaei S, Sharifi I, Mamishi S, Mahmoudvand H (2011) An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis 9(2):67–74. https://doi.org/10.1016/j.tmaid.2011.1002.1003
Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, Akhoundi B, Naeini KM, Avizeh R, Fakhar M (2005) Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol 129(3):243–251. https://doi.org/10.1016/j.vetpar.2005.1001.1010
Neto LDS, Sobrinho LSV, Martins CO, Machado RZ, Marcondes M, de Lima VMF (2011) Use of crude, FML and rK39 antigens in ELISA to detect anti-Leishmania spp. antibodies in Felis catus. Vet Parasitol 177(3): 374–377. https://doi.org/10.1016/j.vetpar.2010.1011.1055
Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, Lorusso E, Gulotta L, Falsone L, Basano FS (2017) Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: pathogen and vector circulation in a confined environment. Vet Parasitol 236:144–151. https://doi.org/10.1016/j.vetpar.2017.1001.1019
Palatnik-de-Sousa CB, Gomes EM, Paraguai-de-Souza E, Palatnik M, Luz K, Borojevic R (1995) Leishmania donovani: titration of antibodies to the fucose-mannose ligand as an aid in diagnosis and prognosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg 89(4):390–393. https://doi.org/10.1016/0035-9203(1095)90022-90025
Palatnik CB, Borojevic R, Previato J, Mendonça-Previato L (1989) Inhibition of Leishmania donovani promastigote internalization into murine macrophages by chemically defined parasite glycoconjugate ligands. Infect Immun 57(3):754–763
Pennisi M-G, Cardoso L, Baneth G, Bourdeau P, Koutinas A, Miró G, Oliva G, Solano-Gallego L (2015) LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors 8(1):302. https://doi.org/10.1186/s13071-13015-10909-z
Pennisi MG (2002) A high prevalence of feline leishmaniasis in southern Italy. Canine Leishmaniasis: moving towards a solution, pp 39–48
Sarkari B, Hatam G, Adnani S, Asgari Q (2009) Seroprevalence of feline leishmaniasis in areas of Iran where Leishmania infantum is endemic. Ann Trop Med Parasitol 103(3):275–277. https://doi.org/10.1179/136485909X136398276
Sarkari B, Pedram N, Mohebali M, Moshfe A, Zargar M, Akhoundi B, Shirzadi M (2010) Seroepidemiological study of visceral leishmaniasis in Booyerahmad district, south-west Islamic Republic of Iran. East Mediterr Health J 16(11):1133–1136
Savani ESMM, de Oliveira Camargo GMC, de Carvalho RM, Zampieri RA, dos Santos MG, D’Áuria SRN, Shaw JJ, Floeter-Winter LM (2004) The first record in the Americas of an autochthonous case of Leishmania (Leishmania) infantum chagasi in a domestic cat (Felix catus) from Cotia County, São Paulo State,Brazil. Vet Parasitol 120(3):229–233. https://doi.org/10.1016/j.vetpar.2004.1001.1008
Sherry K, Miró G, Trotta M, Miranda C, Montoya A, Espinosa C, Ribas F, Furlanello T, Solano-Gallego L (2011) A serological and molecular study of Leishmania infantum infection in cats from the Island of Ibiza (Spain). Vector Borne Zoonotic Dis 11(3):239–245. https://doi.org/10.1089/vbz.2009.0251
Solano-Gallego L, Rodriguez-Cortes A, Iniesta L, Quintana J, Pastor J, Espada Y, Portus M, Alberola J (2007) Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the Northwestern Mediterranean. Am J Trop Med Hyg 76(4):676–680
Trevisan DAC, Lonardoni MVC, Demarchi IG (2015) Diagnostic methods to cutaneous leishmaniasis detection in domestic dogs and cats. An Bras Dermatol 90(6):868–872. https://doi.org/10.1590/abd1806-4841.20153716
Vides JP, Schwardt TF, Sobrinho LSV, Marinho M, Laurenti MD, Biondo AW, Leutenegger C, Marcondes M (2011) Leishmania chagasi infection in cats with dermatologic lesions from an endemic area of visceral leishmaniosis in Brazil. Vet Parasitol 178(1):22–28. https://doi.org/10.1016/j.vetpar.2010.1012.1042
WHO (1990) Control of the leishmaniases. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser, vol 793, pp 1–158
Acknowledgements
Authors would especially like to thank the Vice-chancellor for Research and Technology, Shiraz University of Medical Sciences for their financial support.
Funding
This work is financially supported by Vice-chancellor for Research and Technology, Shiraz University of Medical Sciences grant No. 4856.
Author information
Authors and Affiliations
Contributions
Study was designed by Gholamreza Hatam. Sampling was performed by Qasem Asgari. Material preparation, data collection and analysis were performed by Faeze Foroughi-Parvar. Bahador Sarkari helped to study design and data analysis. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal care and procedures as well as license for blood sampling were under surveillance of Animal Care and Use Committee of Shiraz University of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Foroughi-Parvar, F., Sarkari, B., Asgari, Q. et al. FML-ELISA a novel diagnostic method for detection of feline leishmaniasis in two endemic areas of Iran. J Parasit Dis 45, 279–284 (2021). https://doi.org/10.1007/s12639-020-01316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01316-3