Abstract
Background
Assessing fluid responsiveness is important in the management of patients with hemodynamic instability. Passive leg raising (PLR) is a validated dynamic method to induce a transient increase in cardiac preload and predict fluid responsiveness. Variations in end-tidal carbon dioxide (ETCO2) obtained by capnography correlate closely with variations in cardiac output when alveolar ventilation and carbon dioxide production are kept constant. In this prospective observational study, we tested the hypothesis that variations in ETCO2 induced by a simplified PLR maneuver can track changes in the cardiac index (CI) and thus predict fluid responsiveness.
Method
A five-minute standardized PLR maneuver was performed in 90 paralyzed hemodynamically stable cardiac surgical patients receiving mechanical ventilation. Cardiac index was measured by thermodilution before and one minute after PLR. End-tidal CO2 measurements using capnography were obtained during the entire PLR maneuver. Fluid responsiveness was defined as a 15% increase in the CI. The Chi square test and Student’s t test were used to compare responders and non-responders. Logistic regression analyses were then performed to determine factors of responsiveness.
Results
There were no differences between responders and non-responders in demographic and baseline hemodynamic variables. Fluid responsiveness was associated with an ETCO2 variation (ΔETCO2) of ≥ 2 mmHg during PLR [odds ratio (OR), 7.3; 95% confidence interval (CI), 2.7 to 20.2; P < 0.01; sensitivity 75%]. A low positive predictive value (54%) and a high negative predictive value (NPV) (86%) were observed. No other clinical or hemodynamic predictors were associated with fluid responsiveness. A logistic regression model established that a combination of ΔETCO2 ≥ 2 mmHg and a change in systolic blood pressure ≥ 10 mmHg induced by passive leg raising was predictive of fluid responsiveness (OR, 8.9; 95% CI, 2.5 to 32.2; P = 0.005).
Conclusion
Use of a passive leg raising maneuver to induce variation in ETCO2 is a noninvasive and useful method to assess fluid responsiveness in paralyzed cardiac surgery patients receiving mechanical ventilation. Given its high NPV, fluid responsiveness is unlikely if a passive leg raising maneuver induces ΔETCO2 of < 2 mmHg.
Résumé
Contexte
L’évaluation de la réponse au remplissage est importante dans la prise en charge des patients souffrant d’instabilité hémodynamique. L’élévation passive des jambes (EPJ) est une méthode dynamique validée pour induire une augmentation transitoire de la précharge cardiaque et prédire la réponse au remplissage. Les variations en matière de dioxyde de carbone télé-expiratoire (ETCO2) obtenues par capnographie sont étroitement corrélées aux variations de débit cardiaque lorsque la ventilation alvéolaire et la production de dioxyde de carbone sont maintenues constantes. Dans cette étude observationnelle prospective, nous avons testé l’hypothèse selon laquelle les variations de l’ETCO2 induites par une manœuvre simplifiée d’EPJ peuvent refléter les changements d’index cardiaque (IC) et ainsi prédire la réponse au remplissage.
Méthode
Une manœuvre d’EPJ standardisée de cinq minutes a été réalisée auprès de 90 patients de chirurgie cardiaque hémodynamiquement stables et curarisés recevant une ventilation mécanique. L’index cardiaque a été mesuré par thermodilution avant et une minute après la manœuvre. Les mesures de CO2 télé-expiratoire ont été obtenues par capnographie tout au long de la manœuvre d’EPJ. On a défini la réponse au remplissage en tant qu’une augmentation de 15 % de l’IC. Le test de Chi carré et le test t de Student ont été utilisés pour comparer les répondants et les non-répondants. Des analyses de régression logistique ont ensuite été réalisées afin de déterminer les facteurs de réponse.
Résultats
Aucune différence n’a été observée entre les répondants et les non-répondants en matière de variables démographiques ou hémodynamiques de base. La réponse au remplissage a été associée à une variation d’ETCO2 (ΔETCO2) ≥ 2 mmHg pendant la manœuvre d’EPJ [rapport de cotes (RC), 7,3; intervalle de confiance (IC) 95 %, 2,7 à 20,2; P < 0,01; sensibilité 75 %]. Une valeur prédictive positive basse (54 %) et une valeur prédictive négative élevée (VPN) (86 %) ont été observées. Aucun autre prédicteur clinique ou hémodynamique n’a été associé à la réponse au remplissage. Le modèle de régression logistique a établi qu’une combinaison de ΔETCO2 ≥ 2 mmHg et une modification de la pression artérielle systolique ≥ 10 mmHg induite par une élévation passive des jambes était un prédicteur de réponse au remplissage (RC, 8,9; IC 95 %, 2,5 to 32,2; P = 0,005).
Conclusion
L’utilisation d’une manœuvre d’élévation passive des jambes pour induire des variations d’ETCO2 constitue une méthode non invasive et utile pour évaluer la réponse au remplissage chez des patients de chirurgie cardiaque paralysés recevant une ventilation mécanique. Étant donné sa VPN élevée, la réponse au remplissage est peu probable si une manœuvre d’élévation passive des jambes induit une ΔETCO2 < 2 mmHg.
Similar content being viewed by others
For decades, intravenous fluid administration has played a key role in the management of patients with hemodynamic instability. Uncorrected hypovolemia leads to inappropriate infusion of vasopressors and may result in organ hypoperfusion.1 On the other hand, hypervolemia and excessive fluid administration can lead to increased complications and mortality.2-4 Despite the common use of fluid administration to restore tissue perfusion, studies in the intensive care unit (ICU) have shown that only 50% of hemodynamically unstable critically ill patients are volume-responsive.5 Based on this evidence, there is a need to develop methods to help clinicians assess fluid responsiveness in hemodynamically unstable patients.
Among the tests that have been developed,6 passive leg raising (PLR) is a validated dynamic method to predict fluid responsiveness, and it has proven its reliability in a number of different situations.7 Moving the patient from a semi-recumbent position to a supine position and elevating the patient’s legs to 45o creates a transient endogenous fluid challenge by transferring blood from the lower limbs and the abdominal compartment to the cardiac cavities.8 Passive leg raising increases preload and improves cardiac output if the ventricular function is positioned on the steep part of the Frank-Starling curve. Therefore, a direct measurement of cardiac output is needed in order to assess the response to the PLR maneuver, as surrogate measurements, such as blood pressure and pulse pressure, are unreliable.7 To assess maximal effect, the cardiac output response to PLR must be recorded within 30-90 sec after the onset of the maneuver.8
If monitoring of cardiac output is not available, monitoring of end-tidal carbon dioxide (ETCO2) may represent a useful method to assess the increase in cardiac output with the PLR maneuver. End-tidal CO2 variations correlate closely with cardiac output variations when alveolar ventilation is kept constant -assuming stable production of carbon dioxide.9 This method of monitoring the cardiac output response to PLR has been validated in three recent publications.10-12 In these trials, PLR was standardized using a specialized reclining ICU bed. In certain clinical situations (in the operating room for example), the bed cannot be reclined but the patient’s legs can be lifted from a recumbent position to a 45o angle, creating a partial PLR maneuver.
In this prospective observational study, we tested the hypothesis that a simplified PLR maneuver can induce ETCO2 variation which helps predict fluid responsiveness. In addition, we assessed the various predictors of fluid responsiveness in our patient population.
Methods
Patients
This prospective study was approved by the Ethics Committee of the Montreal Heart Institute in April 2012. Ninety paralyzed adult patients receiving mechanical ventilation and undergoing cardiac or ascending aortic surgery were included in the study. Written informed consent was obtained from every patient prior to surgery. Patients were recruited on the day before their surgery, either on the surgical ward where they were electively admitted or on the medical ward where they were waiting for a semi-urgent procedure. Screening included 108 consecutive consenting patients to achieve a final sample of 90 participants. Exclusion criteria were severe tricuspid regurgitation, lower limb amputation, pregnancy, permanent pacemaker, and presence of atrial fibrillation.
Study design and measurements
Patients were premedicated with oral lorazepam 1-2 mg and subcutaneous morphine 0.1 mg·kg−1 before being taken to the operating room. Additional intravenous midazolam was administered 0.01-0.05 mg·kg−1 in the operating room as needed for patient comfort. Standard cardiac surgery monitoring was used.13 Anesthesia was induced with midazolam 0.04 mg·kg−1 and sufentanil 1 μg·kg−1, and muscle relaxation was achieved with rocuronium 0.8 mg·kg−1. After tracheal intubation, a three-lumen catheter and a thermodilution pulmonary artery catheter were inserted in the right internal jugular vein. Anesthesia was maintained with additional doses of opioids and/or midazolam and/or propofol according to the anesthesiologist’s preference. Inhaled anesthetics were used only for maintenance of anesthesia and not for induction. A transesophageal echocardiography (TEE) examination was performed after induction of general anesthesia. Minute ventilation was adjusted to maintain ETCO2 in the range of 35-45 mmHg with an infrared carbon dioxide analyzer. Volume-controlled ventilation was set for a tidal volume of 6-8 mL·kg−1 with a positive end-expiratory pressure of 5 cm H2O. End-tidal CO2 was continuously measured at the tip of the endotracheal tube using a sidestream infrared gas analyzer connected to the patient’s monitor.
At baseline, the first set of measurements was obtained, including arterial blood pressure, heart rate, central venous pressure (CVP), pulmonary capillary wedge pressure (PCWP), ETCO2, and cardiac index (CI) measured by the indicator dilution method. Cardiac index measurements were obtained by injecting 10 mL of room temperature D5W solution during exhalation and averaging three comparable CI measurements. Values with a variability of > 15% were excluded. A complete TEE exam was also performed by a board certified cardiovascular anesthesiologist. The TEE-based cardiac outputs were not calculated during the PLR maneuver.
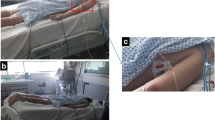
Following baseline measurements, PLR was performed for five minutes by lifting the patient’s legs from a recumbent position to 45o using a standard leg raising device used for sterile preparation of the legs (Fig. 1). The effect of PLR was assessed after one minute, with a repeat measurement of the CI as described previously. As reported in the literature, one minute is considered the time to maximal effect of PLR.8 Meanwhile, heart rate, arterial blood pressure, ETCO2, and CVP were recorded every 12 sec for the five-minute duration of the PLR maneuver. Vasopressors, if needed, were kept constant during this period.
Patients with a > 15% increase in CI attributable to the PLR maneuver were defined as “volume responders”, as previously validated.7,12 Patients with no change or a change of < 15% were defined as “non-responders”. Similarly, an increase in ETCO2 of > 2 mmHg following the PLR maneuver -i.e., the study’s endpoint, was defined as a significant response, identifying volume responders. In three recent trials, a 5% cut-off in ETCO2 variation (ΔETCO2) proved to be reliable for tracking a 15% cardiac output variation following a PLR maneuver.10–12 In these trials, a 5% variation corresponded to a mean absolute value of 2 mmHg.
Statistical analysis
Sample sizes of 28 and 62 patients for responder and non-responder groups, respectively, were sufficient to obtain a power of 0.80 for detecting a 2-mmHg difference in ETCO2 variation when performing a two-sided Student’s t test with a 0.05 significance level. This was assuming a standard deviation of 3.1 mmHg, which was estimated for previous unpublished pilot data. Continuous data are expressed as mean (standard deviation [SD]) and categorical data are expressed as frequency (%). The Student’s t test was used for comparison of hemodynamic parameters between responders and non-responders. For categorical variables, we used the Chi square test. Logistic regression was used to identify factors associated with fluid responsiveness. To calculate the sensitivity and specificity of a ΔETCO2 cut-off of > 2 mmHg as a marker of fluid responsiveness, a receiver operator characteristic curve was constructed. The ETCO2 and cardiac index variations induced by the PLR maneuver were compared using linear regression analysis, and Pearson’s correlation coefficient was calculated. All reported P values are two-sided. Statistical analyses were performed using SAS® 9.3 (Cary, NC, USA).
Results
Ninety patients were studied during April 2012 to May 2013. Following the onset of PLR, 28 patients (31%) were classified as responders and 62 patients (69%) were non-responders. Demographic and surgical data are reported in Table 1. Coronary artery bypass grafting (67%) and aortic valve surgery (33%) were the main interventions performed. There were no significant differences in demographic or surgical parameters between responders and non-responders, except for a higher prevalence of diabetes in the responders group.
Baseline hemodynamic variables
There were no differences in baseline hemodynamic parameters (Table 2) between the two groups, including systolic blood pressure (SBP), mean arterial pressure (MAP), pulse pressure (PP), and CI.
Effects of the PLR maneuver
Hemodynamic response to PLR is shown in Table 3. Fluid responsiveness was associated with an increase in the mean (SD) CI in the responders vs the non-responders group [2.2 (0.5) L·min−1·m−2 vs 1.8 (0.4) L·min−1·m−2, respectively; difference in means, 0.3; 95% confidence interval [CI], 0.1 to 0.5; P ≤ 0.01]. Passive leg raising induced a larger increase in the mean (SD) SBP in the responders vs the non-responders group [12 (17) mmHg vs 2 (13) mmHg, respectively; difference in means, 10; 95% CI, 3 to 16; P ≤ 0.01]. The same was true for the mean (SD) MAP [7 (11) mmHg for responders vs 2 (9) mmHg for non-responders; difference in means, 5; 95% CI, 1 to 9; P = 0.02] and for the mean (SD) PP [7 (9) mmHg for responders vs 1 (8) mmHg for non-responders; difference in means, 7; 95% CI, 3 to 11; P ≤ 0.01]. Mean (SD) absolute values of ETCO2 were not different between responders and non-responders at baseline [36 (3) mmHg for responders vs 36 (3) mmHg for non-responders; difference in means, 0; 95% CI, -1 to 1; P = 0.92] and at one minute after PLR [37 (3) mmHg for responders vs 37 (3) mmHg for non-responders; difference in means, 0; 95% CI, -1 to 2; P = 0.47]. An increase in ETCO2 of > 2 mmHg (ΔETCO2 ≥ 2 mmHg) following the onset of a PLR maneuver was significantly more prevalent in responders than in non-responders (75% vs 29%, respectively; difference in means, 46%; 95% CI, 26 to 66; P ≤ 0.01).
Linear regression analysis of the correlation between the ETCO2 variation and the CI variation following the onset of the PLR maneuver is shown in Fig. 2. A correlation coefficient of 0.47 (95% CI, 0.29 to 0.62; P ≤ 0.01) was calculated.
Prediction of fluid responsiveness
Baseline CVP and PCWP -standard static parameters commonly used by some to predict fluid responsiveness- could not discriminate between fluid responders and non-responders.
Table 4 illustrates the results of logistic regression analyses. An increase of 2 mmHg in ETCO2 (ΔETCO2 ≥ 2 mmHg) was associated with fluid responsiveness [odds ratio (OR), 7.3; 95% CI, 2.7 to 20.2; P < 0.01], with a sensitivity of 75% and an area under the curve of 0.8 (95% CI, 0.7 to 0.9; P < 0.01). Nevertheless, ΔETCO2 ≥ 2 mmHg following the onset of the PLR maneuver was associated with a positive predictive value (PPV) of 54% and a negative predictive value (NPV) of 86%. Following onset of the PLR maneuver, a PP variation (ΔPP) ≥ 9% (OR, 2.4; 95% CI, 0.9 to 5.9; P = 0.06) and an SBP variation (ΔSBP) ≥ 10 mmHg (OR, 2.1; 95% CI, 0.8 to 5.4; P = 0.12) were not significant predictors of fluid responsiveness.
In a logistic regression model (Table 5), a combination of ΔSBP ≥ 10 mmHg and ΔETCO2 ≥ 2 mmHg was a better predictor of fluid responsiveness (OR, 8.9; 95% CI, 2.5 to 32.2; P < 0.01) than each variable independently.
Discussion
This study shows that ETCO2 variation is correlated with changes in cardiac output induced by a simplified PLR maneuver. Thus, it could provide a noninvasive and easily available method at the bedside for predicting fluid responsiveness (or non-responsiveness) in paralyzed patients receiving mechanical ventilation.
End-tidal CO2 is determined by minute ventilation, pulmonary blood flow (i.e., cardiac output), and CO2 production by cell metabolism (VCO2). It is inversely proportional to minute ventilation and proportional to cardiac output and VCO2.9,14 Therefore, assuming constant CO2 production and constant minute ventilation, ETCO2 should reflect changes in cardiac output. This reasoning has been very well described in numerous publications showing a direct and linear relationship between cardiac output and ETCO2 variations. This thinking has been validated for a wide range of cardiac output, including a very low cardiac output state.15–17
Assessing fluid responsiveness is essential in managing hemodynamic instability. The PLR maneuver induces a transient shift of venous blood from the lower limbs and the abdominal compartment towards the heart, increasing right and left cardiac preload.18 Numerous studies,8,19–21 including a recent meta-analysis,7 have shown that the change in cardiac output after PLR is a reliable and accurate method to predict fluid responsiveness.
During PLR, the cardiac output variation must be measured, and this can be achieved noninvasively by obtaining the ETCO2 variation when no direct cardiac output measurement devices are available. Our study shows that a significant change in cardiac output (≥ 15%) induced by PLR is correlated with an ETCO2 variation of > 2 mmHg (ΔETCO2 ≥ 2 mmHg). Therefore, ETCO2 could be considered a surrogate to cardiac output during PLR, predicting fluid responsiveness with a sensitivity of 75%. In a similar trial by Monnet et al., a sensitivity of 71% was obtained.12 Interestingly, before and after PLR, absolute values of ETCO2 were not different between responders and non-responders. This is explained by the fact that ETCO2 is influenced by minute ventilation and VCO2, which are different for every patient included in this study. It shows that ΔETCO2 must be used instead of absolute values. Our study also confirms previous observations7 that SBP and arterial pulse pressure are less reliable than CI to assess the effect of PLR, even if those variables were significantly different between responders and non-responders after the PLR maneuver. Usual static parameters of fluid responsiveness, such as CVP and PCWP, were not reliable in this study. This finding is consistent with observations reported by many groups.22,23
Our study shows that an ΔETCO2 > 2 mmHg induced by PLR is associated with a low PPV (54%), meaning that only 54% of patients with a positive test would be fluid responders. Nevertheless, the high NPV(86%) of this test gives useful information regarding fluid responsiveness. Patients are unlikely to respond to fluids if the ΔETCO2 is < 2 mmHg following the onset of PLR.
These observations confirm the results of three recent and well-designed trials showing accurate tracking of cardiac output by ETCO2 during PLR.10–12 Nevertheless, in these trials, a standard PLR maneuver (semi-recumbent to recumbent position with a 45o elevation of the lower limbs) was performed using a dedicated ICU bed. In certain situations (in the operating room for example), however, only a simplified PLR can be done, starting in a recumbent position and lifting the patient’s legs at a 45o angle. In previous PLR trials, Jabot et al. 24 found significant differences in hemodynamic response to PLR favouring the semi-recumbent starting position, but in a recent meta-analysis by Cavallaro et al.,7 the authors found no difference between those two starting positions. The authors conclude that the hemodynamic effects of PLR are independent from the technique used to perform the maneuver. Fluid responders are on the steep part of their Frank-Starling curve. Only a small fluid challenge is necessary to induce a significant increase in cardiac output25; therefore, a simplified PLR maneuver is sufficient. Someone higher along the Frank-Starling curve, towards the flat part, will not respond, even with a bigger fluid challenge. If fluid is given to these patients, the result will likely be worsening edema.26 In the present study, we showed that fluid responsiveness can be assessed using ETCO2 monitoring and a simplified PLR maneuver.
This prospective observational trial provides a clinically useful way to predict fluid responsiveness using readily available diagnostic tools such as a simplified PLR maneuver and ETCO2 measurement by capnography. In three recent trials, a 5% ΔETCO2 cut-off was proven reliable for tracking a 15% cardiac output variation following a PLR maneuver, which defines fluid responsiveness.10–12 In these trials, a 5% variation corresponded to a mean absolute value of 2 mmHg. For these reasons, a cut-off of 2 mmHg for ΔETCO2 was chosen as a pragmatic and easily applicable hemodynamic target. In clinical practice, absolute values are much easier to use than percentage of variation. Following the same line of reasoning, a 10 mmHg SBP was selected. When we combine these two factors in a logistic regression model, the probability of response to PLR, and fluid loading, is increased. This relation is mostly driven by the presence of an ΔETCO2 ≥ 2 mmHg, which shows once again that CI variation, or its surrogate, is the parameter that must be followed to assess the hemodynamic response to PLR.
End-tidal CO2 monitoring as a surrogate to cardiac output during PLR has some limitations. First, it can help monitor CI variations only during short periods of time since VCO2 is not constant, being dependent on cell metabolism. In situations where the metabolic rate varies considerably, such as in fever or shivering, ETCO2 variations cannot be explained solely by cardiac output variation. Nevertheless, it is unlikely that this phenomenon would be of clinical importance since the maximal hemodynamic effect of PLR is in the first minute. Second, to eliminate fluctuation of minute ventilation, patients must receive volume-controlled ventilation and be paralyzed or deeply sedated to be fully adapted to this mode of ventilation. End-tidal CO2 monitoring would not be reliable with any spontaneous breathing activity. In this study, all patients were anesthetized and paralyzed. The population was also highly selective and might not represent a general intensive care or general surgical population. Nevertheless, they represent patients with cardiovascular disease after the induction of anesthesia before a surgical procedure. With the aging population, this type of patient is likely to increase in the non-cardiac surgical setting. Patients with vasoplegic states or cardiogenic shock were not included in this study, which affects the external validity of this trial. Third, any conditions that affect the interpretation of PLR, such as abdominal hypertension,27 must be taken into account. Fourth, the effect of chronic obstructive pulmonary disease (COPD) on the validity of ETCO2 variation as a marker of cardiac output variation could be questioned. In our study, COPD patients were not excluded, and there was no difference in the number of COPD patients between responders and non-responders, as shown in Table 1. With COPD, ETCO2 is usually lower, reflecting the increase in alveolar dead space. In our population, the mean (SD) basal ETCO2 values were the same in COPD patients [37 (3) mmHg] as in normal subjects [36 (3) mmHg]. Therefore, we can hypothesize that these COPD patients did not have severe disease. When minute ventilation and CO2 production are kept constant, ETCO2 variation correlates closely with cardiac output variation. This is true in both normal subjects and COPD patients. In previous trials by Monnet et al. 12 and Monge Garcia et al.,10 COPD patients were not excluded. Our results correlate with their findings. With regard to the PLR maneuver, there is a lack of studies investigating the validity of this test to predict fluid responsiveness specifically in the COPD population. Finally, continuous cardiac output measurements were not used. Using a standard dilution method, a single measurement was made one minute after the start of the PLR maneuver, i.e., the moment with the maximal reported hemodynamic effect.8 With this protocol, earlier or delayed cardiac output variations might have been missed, incorrectly labelling patients as non-responders.
Conclusion
This study shows that use of a simplified PLR maneuver to induce ETCO2 variation is a noninvasive and useful method to assess fluid responsiveness (positive or negative) in paralyzed cardiac surgery patients receiving mechanical ventilation. Given its high NPV, fluid responsiveness is unlikely if ΔETCO2 < 2 mmHg is observed following PLR.
References
Murakawa K, Kobayashi A. Effects of vasopressors on renal tissue gas tensions during hemorrhagic shock in dogs. Crit Care Med 1988; 16: 789-92.
Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 2006; 34: 344-53.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med 2011; 39: 259-65.
Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol 2010; 6: 107-15.
Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009; 37: 2642-7.
Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 2011; 1: 1.
Cavallaro F, Sandroni C, Marano C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med 2010; 36: 1475-83.
Monnet X, Teboul JL. Passive leg raising. Intensive Care Med 2008; 34: 659-63.
Maslow A, Stearns G, Bert A, et al. Monitoring end-tidal carbon dioxide during weaning from cardiopulmonary bypass in patients without significant lung disease. Anesth Analg 2001; 92: 306-13.
Monge Garcia MI, Gil Cano A, Gracia Romero M, et al. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal CO2 pressure during a passive leg-raising maneuver. Ann Intensive Care 2012; 2: 9.
Young A, Marik PE, Sibole S, Grooms D, Levitov A. Changes in end-tidal carbon dioxide and volumetric carbon dioxide as predictors of volume responsiveness in hemodynamically unstable patients. J Cardiothorac Vasc Anesth 2013; 27: 681-4.
Monnet X, Bataille A, Magalhaes E, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med 2013; 39: 93-100.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia - revised edition 2016. Can J Anesth 2016; 63: 86-112.
Isserles SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg 1991; 73: 808-14.
Shibutani K, Muraoka M, Shirasaki S, et al. Do changes in end-tidal PCO2 quantitatively reflect changes in cardiac output? Anesth Analg 1994; 79: 829-33.
Jin X, Weil MH, Tang W, et al. End-tidal carbon dioxide as a noninvasive indicator of cardiac index during circulatory shock. Crit Care Med 2000; 28: 2415-9.
Idris AH, Staples ED, O’Brien DJ, et al. End-tidal carbon dioxide during extremely low cardiac output. Ann Emerg Med 1994; 23: 568-72.
Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: a technique to measure intravascular volume changes in the capacitance circulation in man. Circulation 1981; 64: 146-52.
Boulain T, Achard JM, Teboul JL, Richard C, Perrotin D, Ginies G. Changes in BP induced by passive leg raising predict response to fluid loading in critically ill patients. Chest 2002; 121: 1245-52.
Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med 2006; 34: 1402-7.
Preau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med 2010; 38: 819-25.
Osman D, Ridel C, Ray P, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 2007; 35: 64-8.
Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002; 121: 2000-8.
Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med 2009; 35: 85-90.
Muller L, Toumi M, Bousquet PJ, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: the mini-fluid challenge study. Anesthesiology 2011; 115: 541-7.
Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth 2014; 112: 617-20.
Mahjoub Y, Touzeau J, Airapetian N, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med 2010; 38: 1824-9.
Funding
Montreal Heart Institute Foundation and the Richard I. Kaufman Endowment Fund.
Conflicts of interest
Dr. André Y. Denault is a Speaker for Covidien and CAE Healthcare and receives royalties from Taylor & Francis.
Author Contributions
Francis Toupin, Ariane Clairoux, Alain Deschamps, and André Y. Denault helped with the study design. Francis Toupin, Ariane Clairoux, Alain Deschamps, Yoan Lamarche, Jean Lambert, Annik Fortier, and André Y. Denault helped with the data analysis. Francis Toupin, Ariane Clairoux, Jean-Sébastien Lebon, and André Y. Denault helped with the conduct of the study and data collection. Francis Toupin, Ariane Clairoux, Alain Deschamps, Jean-Sébastien Lebon, Yoan Lamarche, Jean Lambert, Annik Fortier, and André Y. Denault helped with manuscript preparation. Dr. Denault is the archival author.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Associate Editor, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure
Dr. André Y. Denault: Speakers Bureau for CAE Healthcare and Covidien.
Institutional Review Board Contact
Dr. Philippe Demers, Research and Ethics Committee, Montreal Heart Institute, 5000 Belanger Street, Montreal, QC, Canada H1T 1C8. Phone: 514 376-3330; Fax: 514 376-1355; E-mail: demersph@gmail.com.
Rights and permissions
About this article
Cite this article
Toupin, F., Clairoux, A., Deschamps, A. et al. Assessment of fluid responsiveness with end-tidal carbon dioxide using a simplified passive leg raising maneuver: a prospective observational study. Can J Anesth/J Can Anesth 63, 1033–1041 (2016). https://doi.org/10.1007/s12630-016-0677-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-016-0677-z