Abstract
Purpose
The primary objective of our study was to determine the minimum intravenous dose of carbetocin required to produce adequate uterine contraction in 95% of women (effective dose [ED]95) undergoing elective Cesarean delivery (CD).
Methods
Eighty term pregnant women with low risk for postpartum hemorrhage (PPH) undergoing elective CD under spinal anesthesia were randomly allocated to receive carbetocin intravenously in doses of 80 μg, 90 μg, 100 μg, 110 μg, or 120 μg upon delivery. The consultant obstetrician evaluated the efficacy of the patient’s uterine tone as satisfactory or unsatisfactory. In case of unsatisfactory uterine tone, additional uterotonics were administered as per routine institutional practice. Side effects were monitored during the study period. The main outcome measure was satisfactory uterine tone at two minutes after carbetocin administration.
Results
Satisfactory uterine tone was obtained in 70 subjects (87%) within the dose range of 80-120 μg of carbetocin. It was not possible to calculate the ED95 of carbetocin due to the even distribution of women with satisfactory uterine tone across all dose groups (P = 0.99). Similarly, the side effects were similar across all dose groups. There was a high overall incidence of hypotension (55%) following the administration of carbetocin.
Conclusions
In women at low risk for PPH undergoing elective CD, carbetocin doses of 80-120 μg are similarly effective. There is a high incidence of hypotension associated with carbetocin in these doses, and further studies with doses lower than 80 μg are warranted to assess the balance of efficacy and side effects. This trial was registered at www.clinicaltrials.gov (NCT01262742).
Résumé
Objectif
L’objectif principal de notre étude était de déterminer la dose intraveineuse minimum de carbétocine nécessaire pour obtenir une contraction utérine appropriée chez 95 % des femmes (dose efficace [DE]95) subissant une césarienne programmée.
Méthodes
Quatre-vingts femmes enceintes à terme ayant un faible risque d’hémorragie du postpartum subissant une césarienne programmée sous rachianesthésie ont été randomisées pour recevoir des doses intraveineuses de 80 μg, 90 μg, 100 μg, 110 μg ou 120 μg de carbétocine au moment de l’accouchement. L’obstétricien a évalué l’efficacité du tonus utérin de la patiente comme satisfaisant ou non satisfaisant. En cas de tonus utérin non satisfaisant, des médicaments utérotoniques supplémentaires ont été administrés conformément aux pratiques habituelles de l’établissement. Les effets indésirables ont été surveillés pendant la durée de l’étude. Le critère de jugement principal a été un tonus utérin satisfaisant deux minutes après l’administration de carbétocine.
Résultats
Un tonus utérin satisfaisant a été obtenu chez 70 patientes (87 %) avec une dose de carbétocine comprise entre 80 et 120 μg. Il n’a pas été possible de calculer la DE95 de la carbétocine en raison de la distribution égale des femmes ayant un tonus utérin satisfaisant dans tous les groupes de doses (P = 0,99). Pareillement, les effets indésirables ont été comparables dans tous les groupes thérapeutiques. L’incidence globale de l’hypotension (55 %) a été élevée après l’administration de carbétocine.
Conclusions
Chez des femmes à faible risque d’hémorragie du postpartum subissant une césarienne programmée, des doses de carbétocine de 80 à 120 μg sont pareillement efficaces. Il y a une forte incidence d’hypotension associée à la carbétocine à ces doses et des études supplémentaires sont nécessaires avec des doses inférieures à 80 μg pour évaluer l’équilibre entre efficacité et effets indésirables. Cette étude a été enregistrée sur le site www.clinicaltrials.gov (NCT01262742).
Similar content being viewed by others
Carbetocin [1-deamino-1-carba-2-tyrosine (0-methyl)-oxytocin] is a synthetic analogue of oxytocin that binds to the oxytocin receptors in the myometrium with an affinity similar to that of oxytocin.1,2 It causes an increase in the concentration of calcium that promotes uterine contractility through the same mechanisms as oxytocin.3 Its onset of action is less than two minutes after intravenous or intramuscular administration.4 The main difference between carbetocin and oxytocin is the protracted uterotonic activity of the analogue. This appears to be related to the increased plasma half-life of carbetocin, which is 40 min, approximately four to ten times longer than that of oxytocin.5 This prolonged duration is a result of deamination, which protects carbetocin from the aminopeptidase cleavage, and of the replacement of the disulphide bond by CH2S, which protects the analogue from the disulphidase cleavage.4,5
Hunter et al. 4 assessed the effects of carbetocin in women who had delivered vaginally 24-48 hr prior to the administration of the study drug. The authors found that a single intravenous dose of carbetocin varying from 8-30 μg produced rhythmic uterine contractions lasting for a mean time of 60 min. This long-lasting effect allows carbetocin to be administered as a single dose rather than as a continuous intravenous infusion that is usually required for oxytocin.
In addition to the ease of administration, carbetocin has been shown to decrease the need for additional uterotonic administration when compared with oxytocin infusion after elective Cesarean delivery (CD).6,7 Previous studies showed that women treated with carbetocin 100 μg iv were twice less likely to need additional uterotonic agents when compared with traditional oxytocin regimens.7,8 The incidence and types of side effects between carbetocin and oxytocin have been shown to be similar.8
In 2009, the Society of Obstetricians and Gynaecologists of Canada (SOGC) suggested carbetocin as the first choice of uterotonic agent to prevent postpartum hemorrhage (PPH) after elective CD.9 In lieu of an oxytocin infusion for CD, the SOGC Clinical Practice Guidelines recommend a single dose of carbetocin 100 μg as an intravenous bolus over one minute.9 The justification for the 100 µg dose seems to be based on the fact that most clinical trials to date have used almost exclusively this dose, following the manufacturer’s recommendation. Unpublished data provided by the pharmaceutical company show the results of a study involving 18 women undergoing elective CD wherein none of the women had effective uterine contractions with doses of carbetocin below 60 μg, and 83% (five out of six) developed adequate uterine tone after receiving a dose of 100 μg. The number of patients in this study was small and probably insufficient to establish the recommended dose of 100 μg as a routine practice. Furthermore, in a dose-tolerance study10 wherein carbetocin was administered intramuscularly after vaginal delivery to 45 women, it was suggested that doses up to 200 μg may be safe for clinical use.
The primary objective of our study was to determine the minimum effective intravenous dose of carbetocin required to produce adequate uterine contractions in 95% of women (effective dose [ED]95) at low risk for PPH undergoing elective CD under spinal anesthesia. As secondary objectives, we aimed to identify differences in need for additional uterotonics, estimated blood loss, and side effects across carbetocin dosage groups. Over and above carbetocin dose, we aimed to identify clinical factors associated with inadequate uterine tone at two minutes.
Methods
With the approval of the institutional Research Ethics Board (D-10-01196-A), this double-blind randomized controlled trial was conducted at Mount Sinai Hospital in Toronto. Written informed consent was obtained from all of the participating subjects. Healthy term pregnant women with low risk for PPH and scheduled to have an elective CD under spinal anesthesia were eligible to participate in the study. Exclusion criteria included women with American Society of Anesthesiologists’ physical status III and above, those requiring general anesthesia, and those with conditions predisposing to PPH, such as abnormal placentation, multiple gestation, preeclampsia, macrosomia, polyhydramnios, uterine fibroids, chorioamnionitis, history of uterine atony and PPH, or bleeding diathesis.
The patients were randomized to receive a single intravenous dose of carbetocin, i.e., 80 μg, 90 μg, 100 μg, 110 μg, or 120 μg, using a computer-generated list. Opaque and sealed envelopes containing carbetocin ampoules, dilution instructions, and all equipment necessary for dilution (i.e., syringes, needles, and saline) were then prepared and consecutively numbered. The envelopes were kept refrigerated as per the manufacturer’s recommendationsFootnote 1 and opened just a few minutes prior to the CD. One researcher uninvolved in data collection diluted the study drug in normal saline according to the group allocation to prepare a solution with a final volume of 10 mL. The exact carbetocin dose was drawn from the commercially available carbetocin vial (100 μg·mL−1) using an insulin syringe with 0.1 mL divisions and injected into a 10 mL syringe containing saline to complete the 10 mL volume of the study solution.
Baseline blood pressure (BP) and heart rate (HR) were calculated as the mean of three readings that were taken two minutes apart and recorded in the admitting unit using an automated noninvasive BP device. An 18G peripheral intravenous line was inserted and 10 mL·kg−1 of lactated Ringer’s solution were administered concurrently to the spinal anesthesia placement.
After skin disinfection and local infiltration, a subarachnoid puncture was performed in the sitting position at the L2-3 or L3-4 interspace using a 27G Whitacre needle, and anesthesia was achieved using 0.75% hyperbaric bupivacaine 12-13.5 mg, fentanyl 10 μg, and morphine 100 μg injected over 60 sec. The patient was then immediately positioned supine with left uterine displacement using a wedge under the right buttock. Standard monitoring included electrocardiography, noninvasive BP, HR, and oxygen saturation via pulse oximeter. After the initial co-loading with lactated Ringer’s solution, the rate of fluid administration was reduced to keep the vein open. Blood pressure and HR were recorded every minute until five minutes post delivery and then every 2.5 min. Systolic BP was maintained at baseline levels with aliquots of phenylephrine 0.1 mg iv. Hypotension was defined as a decrease in systolic BP > 20% of the baseline values despite the use of prophylactic phenylephrine. Hypertension was defined as an increase in systolic BP > 20% of baseline values. Bradycardia and tachycardia were defined as a decrease and increase in HR > 30% of baseline respectively.
Immediately upon the delivery of the neonate, the attending anesthesiologist administered the study solution intravenously over one minute as per the recommendation of the manufacturer. The attending anesthesiologist, patient, obstetrician, and investigator collecting the data were blinded to the dose of carbetocin. The obstetrician was requested to allow for spontaneous delivery of the placenta by use of gentle cord traction. No uterine massage was applied within two minutes of study drug administration.
Every minute for five minutes after study drug administration, the obstetrician was asked to assess uterine firmness and to grade its tone as satisfactory or unsatisfactory. The uterine firmness was considered satisfactory if the obstetrician determined there was no need for additional uterotonic agents. In case of unsatisfactory uterine tone and upon the obstetrician’s request, a rescue dose of oxytocin (0.5 IU bolus followed by 40 milliIU·min−1 infusion) or other uterotonics was administered as per our institutional protocol.
The primary aim of the study was to estimate the ED95 for carbetocin based on satisfactory uterine tone at two minutes after its intravenous administration. Secondary outcomes included the use of additional uterotonic agents, estimated blood loss, and side effects (hypo/hypertension, brady/tachycardia, nausea/vomiting, flushing, headache, others) following the carbetocin administration.
Blood loss was estimated by the difference in hematocrit values assessed before and at 24 hr after CD according to the following formula: EBV × (preoperative hematocrit − postoperative hematocrit)/preoperative hematocrit, where EBV indicates estimated blood volume (mL) measured as the patient’s weight (kg) × 85.11
Statistical analysis
The primary aim of this study was to identify the ED95 of carbetocin. There is currently no simple formula to obtain the sample size required to estimate a dose quantile (e.g., ED50 or ED95) with a specified precision.12 To estimate a required sample size with precision around a dose quantile, simulation analyses must be performed using prior data or assumptions to inform the analysis. Consequently, a simple logistic dose-response curve was created using the data provided by the pharmaceutical company from the study with 18 women that resulted in a 0% response rate to 60 μg of carbetocin and an 83% response rate to 100 μg. This prior information was used to construct 100,000 random simulation samples comprised of five treatment groups (doses 80 μg, 90 μg, 100 μg, 110 μg, and 120 μg) of equal size. The sample sizes examined varied from five per group (25 participants) to 50 per group (250 participants). Mean ED95, standard errors, and the corresponding confidence intervals (CI) were calculated for each of these 100,000 samples using Fieller’s method.13 The pilot data suggested an ED95 of 104.5 μg. Based on these assumptions, we expected a confidence interval of ± 6.5 μg around our estimate of ED95 with 15 participants in each dose group. This was considered to represent sufficient clinical precision for dosing. Therefore, to account for 5% loss to follow-up, it was decided to recruit 16 patients in each group for a total of 80 patients. The choice to study a dose range from 80 μg to 120 μg was based on the fact that there was 0% response with doses below 60 μg in the unpublished pilot study, and in a study in which carbetocin was administered intramuscularly after vaginal delivery,10 doses up to 200 μg were considered safe, but doses from 70 to 125 μg were considered optimal.
Demographic and baseline clinical characteristics of the sample are presented according to the intervention arm using means and standard deviations for continuous factors and frequencies and proportions for categorical factors. Due to a monotonic response, the primary aim of estimating the ED95 could not be established. The primary outcome used to inform this analysis (satisfactory uterine contractility at two minutes) was therefore compared across dose groups using Fisher’s exact test. For the secondary outcomes, Kaplan-Meier survival estimates were constructed to examine if time to receive additional uterotonic agents was different across the groups. A log-rank test was used to determine if survival probability differed across groups. One-way analysis of variance was used to compare average estimated blood loss across the groups, and Fisher’s exact tests were used to compare the proportion of patients with side effects across the groups. Further analysis included univariate (simple) logistic regressions conducted to identify potential predictors of treatment failure.
Results
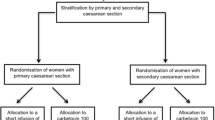
This study was conducted from November 2010 to April 2011. One hundred thirty-one women scheduled for elective CD were approached to participate in the study. Thirty-five patients declined to participate and 16 did not meet the inclusion criteria. Eighty women were randomized to five different dosing groups (i.e., 80, 90, 100, 110, and 120 μg) with 16 subjects in each. All subjects were included in the analysis (Figure).
Preoperative, demographic, and clinical characteristics across the different groups are presented in Table 1.
The overall incidence of women with satisfactory uterine contraction at two minutes after carbetocin administration was 87% (70/80) and did not differ significantly across the various study groups (Table 2). Therefore, it was not possible to create a dose response curve to calculate the ED95 of carbetocin for elective CD, which was the primary aim of this study. Similarly, when the need for additional uterotonic medication within twenty-four hours was analyzed, the results were also monotonic (Table 2). Nine women (11%) required additional uterotonic therapy. In all instances it happened within four hours of carbetocin administration. In the lowest dose (80 μg) group, 12% (2/16) of the women required additional uterotonic drugs, similar to the finding in the 110 μg and 120 μg groups. There was some variation in the 90 μg and 100 μg groups, but this was insufficient to reject the null hypothesis of no difference across the dose groups (P = 0.61). Additional uterotonic agents were required within four hours post-delivery in 60% (6/10) of women who presented with unsatisfactory uterine tone at two minutes as well as in 4% (3/70) of those with initial satisfactory uterine tone. The Kaplan-Meier survival analysis showed no significant differences across the different groups with respect to the number of patients requiring additional uterotonics at any given point in time (P = 0.57).
Finally, we tested to see if there were any differences in estimated blood loss (EBL) across the groups; however, this too was not significant; the mean values were similar across all the groups, with the possible exception of the 110 μg group in which the EBL was higher than in other groups (Table 2). The overall mean (standard deviation) EBL was 648.6 (426.5) mL.
There were no significant differences across the groups in the incidence of side effects related to the use of carbetocin (Table 3). The overall incidence of hypotension was high, about 55%. The other most common side effects encountered were flushing (36%) and nausea (16%). There were no significant differences across the groups in the amount of phenylephrine used after carbetocin administration [F(4,75) = 0.40; P = 0.81]. The overall mean (standard deviation) dose of phenylephrine used post-delivery was 360 (390) μg.
Univariate logistic regressions were conducted to identify potential predictors of carbetocin treatment failure. Given the small number of women with unsatisfactory uterine contractility at two minutes (n = 10), only one predictor variable could be entered into the model at one time. In our sample, there was no single factor that was highly predictive of failure (Table 4).
Discussion
We found satisfactory uterine tone in the majority of the women (87%) with the doses of carbetocin ranging from 80-120 μg; however, it appeared that the efficacy of carbetocin was not dose-dependent within this dose range. No dose tested in our study provided effective uterine contraction in 95% of the women. Interestingly, a small number (4%) of the women with initial satisfactory uterine tone also required additional uterotonic agents. In contrast, the majority of the women (60%) with initial unsatisfactory uterine tone required additional uterotonics. Due to the even distribution of failed treatments across all study groups, it was not possible to create a dose-response curve and to calculate the ED95. It remains unclear whether the dose range studied represents the maximum effective dose of the drug. Consequently, a study is warranted to examine lower doses as there was a high incidence of hypotension across all groups in our study.
The minimum effective dose of oxytocin required to provide adequate uterine contractility at CD has been shown to be less than that traditionally used.14,15 Small intravenous loading doses as low as 0.5 IU provided adequate uterine contractility at elective CD in a population similar to our study.14 Considering that carbetocin is an oxytocin analogue, it is reasonable to assume that 100 μg of carbetocin, which is equivalent to 5 IU of oxytocin according to animal data,4 may also be considerably more than the minimum necessary. This is especially true if the suggestions are accurate that the human uterus is more sensitive to carbetocin than the rat myometrium.1,4
The search for the minimum effective dose of carbetocin is well justified, similar to what has occurred for oxytocin, as carbetocin is thought to have a side effect profile similar to that of oxytocin.6-8,16 The reported side effects of carbetocin during CD include hypotension, headache, nausea, vomiting, tremor, shortness of breath, abdominal pain, back pain, and flushing.6,7,17 Overall, the incidence of these side effects in our study was comparable with previous data.8 In our study, however, there was a high incidence of hypotension.
Although the hemodynamic effects of oxytocin and carbetocin have been described as similar,7,18 the information on the incidence of hypotension following carbetocin administration is not easily found in the literature. Slow boluses or infusions of oxytocin have shown to reduce the cardiovascular effects of this drug.19 Similarly, current recommendations suggest a slow intravenous bolus of carbetocin over one minute.9 , A It is not clear how this one-minute time frame was determined. It appears to be an extrapolation from current clinical practice with oxytocin.
Our study was unable to show a significant difference in the incidence of side effects within the dose-range of 80-120 μg. Given the lack of data in the literature, it is difficult to compare the incidence of hypotension found in our study with other carbetocin studies. Borruto et al. 17 reported a 21.1% incidence of hypotension after administration of carbetocin 100 μg iv during CD, but the authors failed to provide their definition of hypotension. On the other hand, comparing our data with the findings of a similar population that received small doses of oxytocin,14 the current study revealed 55% of hypotension, whereas the oxytocin study showed a 30% incidence. Both studies took place in the same institution, followed nearly identical CD protocols, had a similar patient population, and had the same definition for hypotension. Considering that the doses of oxytocin used in the aforementioned study were small, the apparently higher incidence of hypotension in this study could be partially explained by the fact that the doses used were in fact relatively high and/or by the fact that carbetocin was administered as a bolus, even if a slow one.
One could argue that our protocol was too strict in the definition and recording of hypotensive episodes. Even a single BP reading below 20% of the baseline was considered a hypotensive episode regardless of its transient nature, immediate treatment, or absence of other symptoms. This could explain why the incidence of nausea and vomiting is comparable with previous data8 in spite of the high incidence of hypotension. In addition, all vital signs were computer-captured and recorded. Therefore, it was not left to the discretion of the attending anesthesiologist to report the hypotensive episode. It is worthy of mention that the side effects were secondary outcomes, and the study was not powered to determine the difference in the incidence between groups. In addition, the etiology of complications reported in the context of CD (with the possible exception of flushing) is multifactorial; therefore, the degree of carbetocin’s contribution is difficult to estimate.
Regarding the estimation of blood loss, the comparison of our study with other studies in the literature is problematic for many reasons, including the difference in techniques to estimate blood loss. Boucher et al.6 used a complex technique based on hemoglobin extraction to quantify blood loss during CD. Unfortunately, a comparison with their results is not appropriate as their quantification of blood loss began only after carbetocin administration (mean blood loss of only 159 mL). Borruto et al.17 included both elective and emergency CD in their study and showed a mean blood loss of 600 mL, but the methodology they used was visual estimation. Hematocrit variation calculation used in our study may not be as accurate as hemoglobin extraction techniques, but it provides an assessment free of the subjective bias of visual estimation. Other sophisticated methods for estimation of blood loss can be laborious and expensive.20
Carbetocin at the recommended dose of 100 μg has now been used in thousands of women and has shown to provide adequate uterine contraction with an acceptable safety profile. Nevertheless, it is possible that doses lower than the currently recommended 100 μg may prove advantageous given the theoretical possibility of reducing side effects with the use of lower doses of carbetocin and based on our findings of similar efficacy within the dose range of 80-120 μg. Further studies with doses lower than 80 μg are warranted to assess the balance of efficacy and side effects, namely, hypotension. However, our data may not be extrapolated to other populations, such as women with increased risk for PPH, including those undergoing CD while in labour.
Notes
Ferring Inc. Product monograph: Duratocin (Carbetocin Injection). 2006; 1-20.
References
Atke A, Vilhardt H. Uterotonic activity and myometrial receptor affinity of 1-deamino-1-carba-2-tyrosine(O-methyl)-oxytocin. Acta Endocrinol (Copenh) 1987; 115: 155-60.
Barth T, Krejci I, Kupkova B, Jost K. Pharmacology of cyclic analogues of deamino-oxytocin not containing a disulphide bond (carba analogues). Eur J Pharmacol 1973; 24: 183-8.
Engstrom T, Barth T, Melin P, Vilhardt H. Oxytocin receptor binding and uterotonic activity of carbetocin and its metabolites following enzymatic degradation. Eur J Pharmacol 1998; 355: 203-10.
Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long-acting oxytocin analog on the postpartum uterus. Clin Pharmacol Ther 1992; 52: 60-7.
Sweeney G, Holbrook AM, Levine M, et al. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, in nonpregnant women. Curr Ther Res 1990; 47: 528-40.
Boucher M, Horbay GL, Griffin P, et al. Double-blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. J Perinatol 1998; 18: 202-7.
Dansereau J, Joshi AK, Helewa ME, et al. Double-blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. Am J Obstet Gynecol 1999; 180(3 Pt 1): 670-6.
Su LL, Chong YS, Samuel M. Oxytocin agonists for preventing postpartum haemorrhage. Cochrane Database Syst Rev 2007; 3: CD005457.
Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009; 31: 980-93.
van Dongen PW, Verbruggen MM, de Groot AN, van Roosmalen J, Sporken JM, Schulz M. Ascending dose tolerance study of intramuscular carbetocin administered after normal vaginal birth. Eur J Obstet Gynecol Reprod Biol 1998; 77: 181-7.
Shook PR, Schultz JR, Reynolds JD, Spahn TE, DeBalli P. Estimating blood loss for cesarean section - how accurate are we? Anesthesiology 2003; 98(Supp 1): SOAP A2 (abstract).
Frawley G, Smith KR, Ingelmo P. Relative potencies of bupivacaine, levobupivacaine, and ropivacaine for neonatal spinal anaesthesia. Br J Anaesth 2009; 103: 731-8.
Fieller EC. The biological standardization of insulin. J R Statist Soc 1940; 7(Suppl): 1-64.
Carvalho JC, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: a dose-finding study. Obstet Gynecol 2004; 104: 1005-10.
Balki M, Ronayne M, Davies S, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstet Gynecol 2006; 107: 45-50.
Barton SR, Jackson A. The safety and efficiency of carbetocin to control uterine bleeding following caesarean section. Prenat Neonatal Med 1996; 1: 185.
Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Arch Gynecol Obstet 2009; 280: 707-12.
Attilakos G, Psaroudakis D, Ash J, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double-blind randomised trial. Br J Obstet Gynaecol 2010; 117: 929-36.
Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. Br J Anaesth 2007; 98: 116-9.
Schorn MN. Measurement of blood loss: review of the literature. J Midwifery Womens Health 2010; 55: 20-7.
Acknowledgements
We sincerely thank Mrs. Kristi Downey MSc, Perinatal Research Coordinator at the Department of Anesthesia and Pain Management, Mount Sinai Hospital, for her invaluable contribution in recruiting patients and for creating and maintaining the database. We also thank J. Charles Victor, epidemiologist at the Institute for Clinical Evaluative Sciences, for his support with statistical analysis.
Funding
This study had institutional funding.
Disclosure of interests
The study drug was provided by Ferring Inc., North York, Ontario, Canada.
Details of ethics approval
The study was approved by the Mount Sinai Hospital Research Ethics Board on November 22, 2010 (D-10-01196-A).
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Daniel Cordovani, Mrinalini Balki, Jose C.A. Carvalho, Dan Farine, and Gareth Seaward designed the study. Daniel Cordovani, Mrinalini Balki, and Jose C.A. Carvalho conducted the study, collected and analyzed the data, and wrote the manuscript. Dan Farine and Gareth Seaward reviewed the manuscript.
Rights and permissions
About this article
Cite this article
Cordovani, D., Balki, M., Farine, D. et al. Carbetocin at elective Cesarean delivery: a randomized controlled trial to determine the effective dose. Can J Anesth/J Can Anesth 59, 751–757 (2012). https://doi.org/10.1007/s12630-012-9728-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-012-9728-2