Abstract
Purpose
An airway exchange catheter (AEC) may be employed as a conduit for endotracheal tube placement and for oxygen insufflation or jet ventilation via its lumen. The recent barotrauma-related death of a young healthy patient receiving oxygen insufflated through an AEC prompted the Chief Coroner of Ontario to seek guidelines regarding their use. A literature search was undertaken using a number of search strategies to investigate both the efficacy and complications associated with supplying oxygen through an AEC.
Principal findings
No studies were found comparing either oxygen insufflation or jet-ventilation through an AEC to any standard forms of oxygen therapy. The only case series found using AEC jet ventilation reported that 11% of patients sustained pulmonary barotrauma. Thirteen case reports documented jet ventilation as being associated with pneumothorax, pneumomediastinum, pneumoperitoneum, cardiovascular collapse, and death. In three case series (totalling 76 adults and 20 children) using only oxygen insufflation, no complications were reported.
Conclusions
Jet ventilation through an AEC may be associated with a significant risk of barotrauma. Oxygen insufflation appears to be associated with a lower risk, but it is not risk-free. The authors caution against the use of an AEC to administer oxygen failing the proven benefit of its use over the use of standard oxygen therapies. Should a patient decompensate with an AEC in situ, tracheal re-intubation is the key management strategy. Supplemental oxygen can be provided using standard techniques prior to tracheal intubation or between attempts. Under emergency circumstances, oxygen insufflation or manual ventilation through an AEC may be considered provided vigilance for barotrauma is maintained and re-intubation is not delayed.
Résumé
Objectif
Un échangeur de sonde (AEC) peut être utilisé comme conduit pour positionner une sonde endotrachéale ou pour insuffler de l’oxygène ou de la ventilation en jet par sa lumière. La mort récente, liée à un barotraumatisme, d’un jeune patient en bonne santé recevant de l’oxygène insufflé via un échangeur de sonde a incité le coroner en chef de l’Ontario à demander l’élaboration de directives concernant son utilisation. Une recherche de la littérature a été entreprise à l’aide de plusieurs stratégies de recherche afin d’évaluer l’efficacité et les complications associées à la fourniture d’oxygène via un échangeur de sonde.
Constatations principales
Aucune étude comparant l'insufflation d'oxygène ou la ventilation en jet via un échangeur de sonde à toute méthode standard d'administration d'oxygène n'a été trouvée. L'unique série de cas portant sur l'utilisation d'un échangeur de sonde pour la ventilation en jet a rapporté un barotraumatisme pulmonaire chez 11% des patients. Treize présentations de cas ont décrit la ventilation en jet comme étant associée à des cas de pneumothorax, de pneumomédiastin, de pneumopéritoine, de défaillance cardiovasculaire et de décès. Dans trois séries de cas (pour un total de 76 adultes et 20 enfants) n’ayant recours qu’à une insufflation d’oxygène, aucune complication n’a été rapportée.
Conclusion
L’utilisation d’un échangeur de sonde pour la ventilation en jet pourrait être associée à un risque considérable de barotraumatisme. L’insufflation d’oxygène semble être associée à un risque plus faible, mais elle n’est pas sans risque. Les auteurs émettent une mise en garde concernant les échangeurs de sonde pour administrer de l’oxygène étant donné l’absence de bienfait avéré par rapport aux autres méthodes standard d’administration de l’oxygène. En cas de décompensation d’un patient avec un échangeur de sonde en place, la réintubation trachéale constitue la principale stratégie de prise en charge. L’oxygène supplémentaire peut être administré à l’aide de techniques standard avant l’intubation trachéale ou entre les tentatives. Dans des situations d’urgence, l’insufflation d’oxygène ou la ventilation manuelle via un échangeur de sonde peut être envisagée si l’on reste vigilant pour prévenir tout barotraumatisme et que la réintubation n’est pas retardée.
Similar content being viewed by others
The airway exchange catheter (AEC) or “jet stylet” catheter has been described in the literature in various forms for decades. It is used in a variety of clinical circumstances, e.g., to aid endotracheal tube exchange in an anesthetized patient or to act as a conduit for re-intubation in an awake spontaneously breathing patient during a trial of extubation. Airway exchange catheters are used in a variety of clinical areas, including the operating room and the postanesthetic or intensive care units. Oxygen can be administered through an AEC using either high-pressure or jet ventilation (usually 10-50 psi) or low-pressure variable-flow oxygen (usually 1-10 L·min−1).
The Chief Coroner for Ontario recently reported the death of a 22-yr-old male patient (American Society of Anesthesiologists physical status I) due to tension pneumothorax associated with the use of an AEC.1 The patient had undergone maxillofacial surgery. Intermaxillary fixation with elastic traction was utilized, equivalent to the jaws being “wired shut”. On emergence from anesthesia, an AEC was placed through his nasoendotracheal tube. The nasoendotracheal tube was then removed, leaving the AEC in its place. In addition, a nasogastric tube was placed through the contralateral nostril. The patient was transferred to the postanesthesia care unit (PACU) with the AEC attached to low-pressure oxygen insufflated at a flow rate of 5 L·min−1. Jet ventilation or the provision of oxygen at high pressures was not used. Within minutes of arriving in the PACU, he complained of back pain, and he deteriorated rapidly into cardiopulmonary arrest. Although a needle thoracostomy was performed for a tension pneumothorax, the patient did not survive.
Although jet ventilation through an AEC has been described as potentially resulting in barotrauma, this unusual case of pulmonary barotrauma appeared to result from low-pressure oxygen insufflation alone. With this in mind, we sought to review the efficacy of oxygen supplementation though an AEC compared with oxygen administered by standard modes (e.g., face mask, nasal prongs, continuous positive airway pressure [CPAP], and bi-level positive airway pressure [BiPAP]) as well as the associated complications. This report does not address the utility of AECs for tracheal re-intubation.
Search strategy
A literature review was undertaken seeking to determine the efficacy and complications of supplying oxygen through an AEC. Four separate databases were used in the search: MEDLINE®, EMTREE (Embase™), HealthSTAR, and CINAHL®. Our strategy included reviewing all articles with the terms “airway exchange catheter” and/or “jet stylet” as keywords. These terms were also combined with the subject heading or keyword search (depending on the database) of “pneumothorax”, “barotrauma”, “lung injury”, and “volutrauma”. All retrieved articles were hand searched for further relevant references.
Principal findings
The effectiveness of an AEC for oxygenation
In our search of the four separate databases, no randomized controlled trials were found comparing jet ventilation or oxygen insufflation through an AEC with standard modes of providing oxygen. Therefore, we could not determine the benefit of supplementing oxygen through an AEC vs other standard oxygen therapies.
Table 1 includes all identified case series reporting the use of oxygen through an AEC. In the reported series, oxygen supplementation through the AEC was not the primary study outcome; generally, it was the overall utility of maintaining tracheal access following extubation of patients who would be difficult to re-intubate. One larger case series reporting AEC use2 was excluded because it made no mention of oxygen supplementation through the AEC.
Oxygen insufflation
Oxygen insufflation at rates of 1-8 L·min−1 was reported in two adult series totalling 76 patients.3,4 In a further series of 20 pediatric patients, oxygen insufflation was used at a flow rate of 2-6 L·min−1.5 Patients in all three series were breathing spontaneously, and where reported,4,5 none suffered oxygen desaturation < 90%. No cases of barotrauma were reported despite the adult studies being conducted on postoperative patients with known upper airway swelling. In a separate pediatric case series of 11 infants less than 97 days old, Fayoux6 reported using either manually-assisted or volume-controlled ventilation through an AEC during laryngotracheal stenosis surgery. One neonate became hypoxemic and required tracheotomy, but none suffered barotrauma.
Jet ventilation
In the only case series we found that made mention of jet ventilation through an AEC, Cooper7 reported on 45 patients who received jet ventilation through a CardioMed endotracheal ventilation catheter (ETVC) (Lindsay, ON, Canada). Thirty of these patients were anesthetized and pharmacologically paralyzed for various surgical procedures involving the lower airway. No data were presented in the series regarding the efficacy of oxygenation or ventilation.
Complications associated with oxygen supplementation through an AEC
Table 2 includes a summary of the literature reporting complications associated with oxygen supplementation through an AEC. Barring one case series by Cooper,7 most publications were in the form of case reports. In Cooper’s case series,7 barotrauma occurred in five of 45 patients (11%) who received jet ventilation using driving pressures of 15-50 psi. Although not mentioned directly in his article, the authors have confirmed with Dr. Cooper that all cases of barotrauma were pulmonary in origin (personal communication, 2011). A further thirteen cases of barotrauma were found associated with oxygenation through an AEC.8-14 Sixteen (89%) of the 18 patients who sustained barotrauma or volutrauma had received jet ventilation. Of those jet ventilated, thirteen (81%) sustained pulmonary barotrauma; two patients suffered gastrointestinal barotrauma,10,12 and one patient manifested pulmonary volutrauma.12
Barotrauma has been reported over a wide range of jet ventilation driving pressures, including pressures as low as 15 psi.7,9
Does pulmonary barotrauma occur in the setting of oxygen insufflation through AECs without jet ventilation? The case detailed in the Coroner’s report1 suggests the answer is yes. Two other case reports (included in Table 2) describe barotrauma without jet ventilation. In one case, oxygen insufflation through a misplaced esophageal AEC led to gastric rupture within one hour of initiating therapy.15 In the other case, manual ventilation through an AEC caused a pneumothorax.16 Conversely, no patients suffered barotrauma in the abovementioned three case series involving 76 adult3,4 and 20 pediatric spontaneously breathing patients5,6 in whom oxygen insufflation was used at flow rates of 1-8 L·min−1. In Dosemeci’s study,3 18 of 36 patients had an AEC placed in the contralateral nostril along with a nasogastric tube (similar to the case described by McCallum1) yet no patient suffered barotrauma. This result suggests a lesser risk of volutrauma or barotrauma during oxygen insufflation through an AEC compared to jet ventilation.
In a separate case report of a right tension pneumothorax in a patient using jet ventilation, Baraka suggested oxygen should be maintained through the AEC at a flow rate of no more than 1-2 L·min−1.8 However, given the other cases reported and the one described in this communication, even this flow rate may not be innocuous; it does not take into account the issue of impaired exhalation of tidal volume nor is it based on evidence.
Discussion
Structure and function of airway exchange catheters
The primary role of an AEC is to act as a “place holder” to maintain tracheal access while one ETT is exchanged for another. This can occur either immediately (e.g., when a double-lumen tube is exchanged for a single-lumen ETT), or it can occur when there is a possibility that an ETT may need to be re-inserted after extubation, particularly when earlier the patient had been difficult to intubate (i.e., “trial of extubation”). On occasion, the AEC is used as a conduit to administer oxygen. When used to retain tracheal access upon extubation, an AEC of 11Fr or 14Fr diameter is well-tolerated in over 90% of awake adult patients,2 as long as it is not advanced to or beyond the highly innervated carina (Fig. 1). Although appropriate for adult tube exchanges, the larger 19Fr Cook AEC is associated with a much higher rate of patient discomfort2 and, as such, is used rarely in the “trial of extubation” scenario.
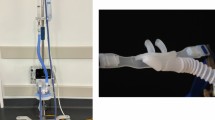
An awake patient in the postanesthesia care unit with an 11Fr, 83-cm Cook airway exchange catheter (AEC) in situ following anterior cervical spine fusion. Note supplemental oxygen administration via face mask. In this case, the AEC is routed through the face mask and taped to both the mask and forehead. The patient is able to phonate and breathe easily. The patient granted consent for publication of the masked image file
The most popular airway exchange catheters are made by Cook Medical (Bloomington, Indiana, www.cookmedical.com). Available in a variety of diameters and lengths (Table 3), they are hollow with two side ports proximal to the distal opening, which are designed to help prevent obstruction to oxygen insufflation or jet ventilation. Sizes 8Fr and 11Fr AECs are usually considered “pediatric” because of the minimum-sized ETT they can accommodate. Variations to the standard Cook AECs include longer stiffer AECs that are designed to facilitate double-lumen tube exchanges. The ETVC from CardioMed is available in a single size. Both the Cook and CardioMed products are supplied with proximal connectors (e.g., Cook Medical’s Rapi-Fit® adaptor) to allow the option of oxygenation through the catheter. Airway exchange catheters are also produced and marketed by other companies.
Oxygen supplementation through an AEC
Oxygenation through an AEC usually occurs during one of two clinical scenarios: 1) Passage of the replacement ETT has failed during an ETT exchange or re-intubation facilitated by an AEC, and oxygen is jetted or insufflated pending a decision on how to proceed; or 2) A patient with a known or potentially difficult airway has been extubated over an AEC, and oxygen insufflation is performed electively through the catheter.
Oxygen supplementation is accomplished through the hollow lumen of an AEC via the supplied adapters (Fig. 2) in one of two ways:
Rapi-Fit® adaptors fitting the proximal end of the Cook airway exchange catheter (AEC). The Cook AEC is packaged with the Luer-Lok® adaptor already attached for jet ventilation or oxygen insufflation. Alternatively, a standard 15-mm adaptor is also supplied for oxygen insufflation or positive pressure ventilation
-
1)
Using low-pressure variable-flow oxygen at 1-15 L·min−1. This is associated with low driving pressures even at the higher range of flow. Oxygen delivery can be accomplished through the lumen of the AEC using the 15-mm Rapi-Fit adapter (Cook Medical, Bloomington, IN, USA) either by intermittent positive pressure ventilation or by continuous oxygen insufflation.
-
2)
Using high-pressure oxygen or “jet-ventilation”, usually with a driving pressure of 15-50 psi utilizing the Luer-Lok® adaptor. Hospital pipeline oxygen is supplied at approximately 50 psi, which may be reduced using a variety of down-regulating devices. The oxygen flow produced depends on a complex dynamic interaction of several factors, including driving pressure, inspiratory time, lung compliance, AEC length, and lumen size.17
The product monograph regarding oxygenation through the Cook Medical AECs25 includes the following statements:
-
A ventilatory device may be used at any time during the ETT exchange procedure by utilizing the attached Rapi-Fit adaptor;
-
Potential adverse events include barotrauma and perforation of the bronchi or lung parenchyma;
-
To avoid barotrauma, ensure that the tip of the AEC is always above the carina, preferably by 2-3 cm;
-
If a high-pressure oxygen source is used for insufflation (e.g., jet ventilator), begin at lower pressure and work up gradually. Rising chest wall, pulse oximetry, and oral air flow should be carefully monitored.
Mechanisms of barotrauma
Pulmonary barotrauma is a term well understood amongst medical professionals. Rapid or excessive application of positive pressure to the tracheobronchial tree, as may occur with mechanical or jet ventilation, can damage respiratory structures. The term “volutrauma” is a relative newcomer to the medical lexicon, having originated in the critical care literature in the early 1990s.18 This distinct entity implies an overdistension of alveoli, which leads to alveolar injury, increased mean intrathoracic pressure, impeded venous return, and other adverse events. At some point, the increase in volume leads to an increase in pressure that may culminate in barotrauma and its pathophysiologic sequelae (e.g. tension pneumothorax, air embolism, and others).
More simply, while high pressure jet ventilation may cause “pure” barotrauma by direct damage to tissues,19 under certain circumstances, low or moderate flow gas insufflation may lead initially to volutrauma and ultimately to barotrauma. Continuous gas insufflation into the tracheobronchial tree is particularly hazardous in the face of upper airway obstruction that may hinder egress of the insufflated gas volume. If the volume of exhaled gas is less than the volume of inhaled gas, whether the inspired gas is jetted or insufflated through an AEC will not determine whether volutrauma or barotrauma will occur, it will simply affect the timeline it takes to occur. Thus, jet ventilation is not required for lethal barotrauma to occur while using an AEC.
Use of the AEC for oxygenation: an historical perspective
The 1993 and 2003 American Society of Anesthesiologists Practice Guidelines for the Management of the Difficult Airway20,21 suggested that an “intratracheal jet stylet” is useful in a difficult ventilation situation and be included in the contents of a difficult airway cart.
In the early 1990s, Benumof carried out much of the initial research regarding the use of the AEC for jet oxygenation using a mechanical lung model. Prior to the widespread use of the laryngeal mask airway as a rescue device, instead of moving directly to surgical airway, transtracheal and translaryngeal jet ventilation were investigated as a means to provide oxygenation and ventilation in the “can’t intubate, can’t ventilate” failed airway situation.
Research focused on measuring the inspiratory tidal volumes generated by jet ventilation through an AEC and varying factors, such as the driving pressure, insufflation time, stylet resistance, and “lung” compliance. Benumof et al. operated the mechanical lung model with a driving pressure of 50 psi and normal “lung” compliance, and they found that tidal volumes of 440-1,680 mL were produced in one second by varying the size of the AEC.17 Entrainment of additional air around the AEC (the Venturi effect) was relatively non-contributory, as the AEC was placed well below the glottis.22 Although Benumof originally advocated 50 psi when jetting through an AEC, he later revised this to start at a pressure of “less than 50 psi”.23
Benumof et al. used the mechanical lung model once again to study the influence of tracheal diameter.24 They found that air-trapping took place consistently when the tracheal diameter was < 4.0-4.5 mm. As a mechanical model cannot account for soft tissue compliance, this “critical diameter” may vary with the unique compliance of the obstructing anatomy in an individual patient. Whether a patient is breathing spontaneously or is assisted, the exhaled tidal volume will escape using the path of least resistance - mainly around, and not through, the AEC. Therefore, the external diameter of the AEC will impact the airway diameter required for adequate expiration. As Benumof stated, “If the equivalent annular air exit space that surrounds the AEC is less than a 4-mm internal diameter ETT, ventilation will not be possible because exhalation time will become markedly prolonged.”22
Calculating inspired volumes produced with jet ventilation by determining and weighting several variables is only one side of the equation. In clinical practice, probably the most important aspect of the formula is to ensure that inspiratory and expiratory volumes remain equal. Complete exhalation must occur before the next inspiratory cycle.14 While clinically determining “complete exhalation” may seem straightforward on the surface, in reality, it is close to impossible, particularly in the setting of a patient not on a mechanical ventilator, i.e., the typical patient in whom an AEC is placed. Although the Cook Medical product insert for the AEC suggests careful monitoring of “rising chest wall, pulse oximetry, and oral air flow”,25 equally or perhaps more important is observing the patient for a falling chest wall, which would reflect adequate exhalation. Candido19 observed, “There is simply not any monitoring device that can quantify ventilation with any degree of certainty”.
Adding to this complexity is phonation; when an AEC is used during a trial of extubation, patients can and do speak with an AEC in place.7,12 Benumof recommended that the patient not phonate if jet ventilation is required.22 Since the glottic opening is the narrowest part of the airway,26 any adduction of the vocal cords during jet ventilation could lead to air trapping and barotrauma. This issue was highlighted in a patient case report by Egol12 in 1985. The patient had his jaws wired shut postoperatively after surgical reduction for prognathism. A nasogastric tube was intentionally placed nasotracheally to act as an AEC. Although the patient was stable, a “demonstration trial” of jet ventilation was undertaken in the PACU. The initial jet settings were 20 psi with an inspiration:expiration (I:E) ratio of 1:2. The patient remained comfortable “until he tried to talk”. Egol reported, “At that time, his mean arterial blood pressure fell rapidly to 30 mmHg, presumably due to closure of his vocal cords around the catheter.” A chest x-ray revealed no evidence of barotrauma.
Logically, if vocal cord adduction for brief periods of time during jet ventilation results in hypotension, then prolonged adduction, as in the setting of laryngospasm or other obstructing laryngeal pathology (e.g., significant edema), could also lead to volutrauma or barotrauma, even with low-flow insufflation. Unless the degree of auto-positive end-expiratory pressure (auto-PEEP) can be measured or the patient explicitly demonstrates respiratory distress or signs of increased intrathoracic pressure, gradually increasing intrathoracic pressure may go unnoticed until the patient suddenly deteriorates.
Conclusion
There is no literature comparing either oxygen insufflation or jet-ventilation through an AEC to any standard oxygen delivery modalities. It follows that there is no evidence showing either jet-ventilation or oxygen insufflation via an AEC provides better oxygenation than that afforded by standard therapies.
As reflected by case series and reports, jet ventilation through an AEC over a wide range of driving pressures and clinical conditions appears to be associated with a clinically significant risk of barotrauma. Although there have been several cautionary editorials22,23,27 regarding the need to assess the degree of airway patency around the AEC to allow for passive exhalation, this is very difficult to determine at the bedside. Similarly, cautions regarding the need for maintaining an AEC in the mid-tracheal position and initiating jet ventilation at lower driving pressures are also problematic; both circumstances have been associated with barotrauma. Oxygen insufflation through an AEC appears to be associated with a lower risk of volutrauma or barotrauma, but, as suggested by the case presented by the Ontario Coroner, it is not risk-free. Until there is proven benefit of supplying oxygen through an AEC vs standard oxygen therapies, the authors would caution against its routine use.
Should a patient with an AEC in situ decompensate, tracheal re-intubation is the key management strategy. Supplemental oxygen can be provided using standard techniques prior to intubation. Under emergency circumstances, oxygen insufflation or manual ventilation through an AEC may be considered provided vigilance for barotrauma is maintained and tracheal intubation is not delayed.
References
McCallum AL. Airway Exchange Catheters (AEC)/Endotracheal Ventilation Catheter (ETVC), September 16, 2010. Available from URL: http://www.cas.ca/English/Page/Files/109_coroner_letter.pdf (accessed March 2011).
Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg 2007; 105: 1357-62.
Dosemeci L, Yilmaz M, Yegin A, Cengiz M, Ramazanoglu A. The routine use of pediatric airway exchange catheter after extubation of adult patients who have undergone maxillofacial or major neck surgery: a clinical observational study. Crit Care 2004; 8: R385-90.
Loudermilk EP, Hartmannsgruber M, Stoltzfus DP, Langevin PB. A prospective study of the safety of tracheal extubation using a pediatric airway exchange catheter for patients with a known difficult airway. Chest 1997; 111: 1660-5.
Wise-Faberowski L, Nargozian C. Utility of airway exchange catheters in pediatric patients with a known difficult airway. Pediatr Crit Care Med 2005; 6: 454-6.
Fayoux P, Marciniak B, Engelhardt T. Airway exchange catheters use in the airway management of neonates and infants undergoing surgical treatment of laryngeal stenosis. Pediatr Crit Care Med 2009; 10: 558-61.
Cooper RM. The use of an endotracheal ventilation catheter in the management of difficult extubations. Can J Anaesth 1996; 43: 90-3.
Baraka AS. Tension pneumothorax complicating jet ventilation via a cook airway exchange catheter. Anesthesiology 1999; 91: 557-8.
Chang JL, Bleyaert A, Bedger R. Unilateral pneumothorax following jet ventilation during general anesthesia. Anesthesiology 1980; 53: 244-6.
Chang JL, Meeuwis H, Bleyaert A, Babinski M, Petruscak J. Severe abdominal distention following jet ventilation during general anesthesia. Anesthesiology 1978; 49: 216.
Cooper RM, Cohen DR. The use of an endotracheal ventilation catheter for jet ventilation during a difficult intubation. Can J Anaesth 1994; 41: 1196-9.
Egol A, Culpepper JA, Snyder JV. Barotrauma and hypotension resulting from jet ventilation in critically ill patients. Chest 1985; 88: 98-102.
Mcdonald DS, Liban JB. A serious complication with an airway exchange catheter. Clinical Intensive Care 1997; 8: 36-7.
Nunn C, Uffman J, Bhananker SM. Bilateral tension pneumothoraces following jet ventilation via an airway exchange catheter. J Anesth 2007; 21: 76-9.
Fetterman D, Dubovoy A, Reay M. Unforeseen esophageal misplacement of airway exchange catheter leading to gastric perforation. Anesthesiology 2006; 104: 1111-2.
Chen WY, Lin JA, Chen HL, Wong CS, Ho ST, Lu CC. Pneumothorax associated with tube exchanger-aided intubation following LMA-Fastrach placement in a patient during anesthesia induction. Acta Anaesthesiol Taiwan 2004; 42: 227-31.
Gaughan SD, Benumof JL, Ozaki GT. Quantification of the jet function of a jet stylet. Anesth Analg 1992; 74: 580-5.
Dreyfuss D, Saumon G. Barotrauma is volutrauma, but which volume is the one responsible? Intensive Care Med 1992; 18: 139-41.
Candido KD, Saatee S, Appavu SK, Khorasani A. Revisiting the ASA guidelines for management of a difficult airway. Anesthesiology 2000; 93: 295-8.
Anonymous. Practice guidelines for management of the difficult airway. A report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 1993; 78: 597-602.
American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003; 98: 1269-77.
Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Anesthesiology 1999; 91: 342-4.
Benumof JL, Gaughan SD. Concerns regarding barotrauma during jet ventilation. Anesthesiology 1992; 76: 1072-3.
Dworkin R, Benumof JL, Benumof R, Karagianes TG. The effective tracheal diameter that causes air trapping during jet ventilation. J Cardiothorac Anesth 1990; 4: 731-6.
Cook Airway Exchange Catheters with RapiFit® Adapters - Instructions for Use. ©Cook Medical 2010. Available from URL: www.cookmedical.com/cc/resources.do?id=4807 (accessed February 2011).
Morris IR. Preparation for awake intubation. In: Hung O, Murphy MF, editors. Management of the difficult and failed airway. 1st ed. New York: McGraw-Hill; 2007. p. 40.
Benumof JL. Airway exchange catheters for safe extubation: the clinical and scientific details that make the concept work. Chest 1997; 111: 1483-6.
Acknowledgements
The authors sincerely thank the Chief Coroner of Ontario, Dr. Andrew McCallum, for bringing this case to the attention of the Canadian Anesthesiologists’ Society. We also thank Dr. Harry Foster, Coroner and Anesthesiologist, Sunnybrook Hospital, Toronto. We are grateful to Ms. Brooke Ballantyne Scott MLIS for her helpful assistance to focus our literature search, and we thank Dr. Richard M. Cooper and Dr. Thomas C. Mort for providing more information regarding their studies. Finally, we thank Drs. Orlando Hung and Ron M. Walls for their review and suggestions during the initial stages of writing our manuscript.
Disclosure
Authors have received no funding from any source and have no commercial or non-commercial affiliations with the companies mentioned in this work.
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duggan, L.V., Law, J.A. & Murphy, M.F. Brief review: Supplementing oxygen through an airway exchange catheter: efficacy, complications, and recommendations. Can J Anesth/J Can Anesth 58, 560–568 (2011). https://doi.org/10.1007/s12630-011-9488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-011-9488-4