Abstract
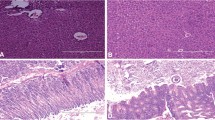
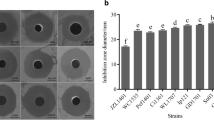
Bacteriocin TSU4 is a novel antimicrobial peptide isolated from Catla catla gut isolate Lactobacillus animalis TSU4. It has been reported for its potential antimicrobial activity against fish pathogens and food spoilage bacteria. In vivo safety evaluation is necessary to determine its immunogenicity, toxicity, and importance in real-life applications. The present study was designed to evaluate the immunogenicity, acute and sub-chronic toxicity of bacteriocin TSU4 in BALB/c mice to ensure its safety in industrial application. Male BALB/c mice were administered intraperitoneally for immunogenicity assessment, by oral gavage with 50, 100, and 200 mg/kg/body weight for acute test and 0.5 mg/kg/day dose of bacteriocin TSU4 for sub-chronic toxicity test. Neither mortality nor any infections were observed during experimental period. There was no major increase in antibody titer during the immunogenicity test, and no mortality was observed during acute or sub-chronic toxicity tests. The LD50 value of bacteriocin TSU4 was found to be higher than 200 ± 0.45 mg/kg. No significant change in the serum biochemical markers, histopathological analysis and visual observation in spleen sizes was observed. These findings revealed that bacteriocin TSU4 is a non-immunogenic, safe, non-toxic, and could be a potential candidate for industrial applications in food preservation and aquaculture industries.






Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- BC:
-
Bowman’s capsule
- BLS:
-
Bacteriocin-like substance
- body wt.:
-
Body weight
- CV:
-
Central vein
- D:
-
Distal convoluted tubule
- G:
-
Glomerulus
- KC:
-
Kupffer cells
- L. :
-
Lactobacillus
- LB:
-
Luria Bertani
- LD:
-
Lethal dose
- MRS:
-
deMan Rogosa Sharpe
- P:
-
Proximal convoluted tubule
- PBS:
-
Phosphate buffer saline
- SGOT:
-
Serum glutamic oxaloacetic transaminase
- SGPT:
-
Serum glutamic pyruvic transaminase
References
Chen YS, Wang YC, Chow YS, Yanagida F, Liao CC, Chiu CM (2014) Purification and characterization of plantaricin Y, a novel bacteriocin produced by Lactobacillus plantarum 510. Arch Microbiol 196(3):193–199. doi:10.1007/s00203-014-0958-2
Gupta R, Sarkar S, Srivastava S (2014) In vivo toxicity assessment of antimicrobial peptides (AMPs LR14) derived from Lactobacillus plantarum strain LR/14 in Drosophila melanogaster. Probiotics & Antimicro Prot 6(1):59–67. doi:10.1007/s12602-013-9154-y
Casaburi A, Martino AD, Ferranti P, Picariello L, Villani F (2016) Technological properties and bacteriocins production by Lactobacillus curvatus 54 M16 and its use as starter culture for fermented sausage manufacture. Food Control 59:39–49. doi:10.1016/j.foodcont.2015.05.016
Nguyen C, Nguyen VD (2016) Discovery of azurin-like anticancer bacteriocins from human gut microbiome through homology modeling and molecular docking against the tumor suppressor p53. Biomed Res Int. doi:10.1155/2016/8490482
Nghe D, Nguyen T (2014) Characterization of antimicrobial activities of Pediococcus pentosaceus Vtcc-B-601. J Appl Pharma Sci 4(5):61–64. doi:10.7324/JAPS.2014.40511
Arthur TD, Cavera VL, Chikindas ML (2014) On bacteriocin delivery systems and potential. Future Microbiol 9(2):235–248. doi:10.2217/fmb.13.148
Yang SC, Lin CH, Sungs CT, Fang JY (2014) Antibacterial activities of bacteriocins: application in food and pharmaceuticals. Front Microbiol 5:241. doi:10.3389/fmicb.2014.00241
Kanagaraj J, Selvi AT, Senthilvelan T, Chandra babu NK, Chandrasekar B (2014) Evaluation of new bacteriocin as a potential short-term preservative for goat skin. Am J Microbiol Res 2(3):86–93. doi:10.12691/ajmr-2-3-2
Jena PK, Trivedi D, Purandhar K, Chaudhary H, Seshadri S (2011) Antimicrobial peptides and its role in gastro-intestinal diseases: a review. J Anim Sci Adv 1(1):1–11
Borges S, Barbosa J, Silva J, Teixeira P (2014) Characterization of a bacteriocin of Pediococcus pentosaceus SB83 and its potential for vaginal application. Anti-Infective Agents 12(1):68–74
Sahoo TK, Jena PK, Patel AK, Seshadri S (2016) Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: a review. Aquac Res 47(4):1013–1027. doi:10.1111/are.12556
Stoyanova LG, Sultimova TD, Botina SG, Netrusov AI (2006) Isolation and identification of new nisin producing Lactobacillus lactis subsp. lactis from milk. Appl Biochem Microbiol 42(5):492–499. doi:10.1134/S0003683806050085
Jena PK, Trivedi D, Chaudhary H, Sahoo TK, Seshadri S (2013) Bacteriocin PJ4 active against enteric pathogen produced by Lactobacillus helveticus PJ4 isolated from gut microflora of Wistar rat (Rattus norvegicus): partial purification and characterization of bacteriocin. Appl Biochem Biotech 169(7):2088–2100. doi:10.1007/s12010-012-0044-7
Trivedi D, Jena PK, Patel JK, Seshadri S (2013) Partial purification and characterization of a bacteriocin DT24 produced by probiotic vaginal Lactobacillus brevis DT24 and determination of its anti-uropathogenic Escherichia coli potential. Probiotics & Antimicro Prot 5(2):142–151. doi:10.1007/s12602-013-9132-4
Rivas FP, Castro MP, Vallejo M, Marguet E, Campos CA (2014) Sakacin Q produced by Lactobacillus curvatus ACU-1: functionality characterization and antilisterial activity on cooked meat surface. Meat Sci 97(4):475–479. doi:10.1016/j.meatsci.2014.03.003
Mioa J, Guo H, Ou Y, Liu G, Fang X, Liao Z, Ke C, Chen Y, Zhao L, Cao Y (2014) Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet, China. Food Control 42:48–53. doi:10.1016/j.foodcont.2014.01.041
Maldonado-Barragan A, Caballero-Guerrero B, Martin V, Ruiz-Barba JL, Rodriguez JM (2016) Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol 17:37. doi:10.1186/s12866-016-0663-1
Todorov SD, Rachman C, Fourrier A, Dicks LM, van Reenen CA, Prevost H, Dousset X (2011) Characterization of a bacteriocin produced by Lactobacillus sakei R 1333 isolated from smoked salmon. Anaerobe 17(11):23–31. doi:10.1016/j.anaerobe.2010.01.004
Paiva AD, Fernandes KM, Dias RS, Rocha A, Dos S, de Oliveira LL, Neves CA, de Paula SO, Mantovani HC (2013) Safety evaluation of the antimicrobial peptide bovicin HC5 orally administered to a murine model. BMC Microbiol 13:69. doi:10.1186/1471-2180-13-69
Wadhwa M, Knezevic I, Kang HN, Thorpe R (2015) Immunogenicity assessment of biotherapeutic products: an overview of assays and their utility. Biologicals 43(5):298–306. doi:10.1016/j.biologicals.2015.06.004
Pariza MW, Foster EM (1983) Determining the safety of enzymes used in food processing. J Food Prot 46:453–468
Sahoo TK, Jena PK, Nagar N, Patel AK, Seshadri S (2015a) In vitro evaluation of probiotic properties of lactic acid bacteria from the gut of Labeo rohita and Catla catla. Probiotics & Antimicro Prot 7(2):126–136. doi:10.1007/s12602-015-9184-8
Sahoo TK, Jena PK, Patel AK, Seshadri S (2015b) Purification and molecular characterization of the novel highly potent bacteriocin TSU4 produced by Lactobacillus animalis TSU4. Appl Biochem Biotech 177(1):90–104. doi:10.1007/s12010-015-1730-z
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7(1–2):248–254
Vaucher R, De A, Gewehr C, De CV, Correa APF, Santanna V, Ferreira J, Brandelli A (2011) Evaluation of the immunogenicity and in vivo toxicity of the antimicrobial peptide P34. Int J Pharm 421(1):94–98. doi:10.1016/j.ijpharm.2011.09.020
FDA (Food Drug Administration) (1988) Nisin preparation: affirmation of GRAS status as direct human food ingredient. Fed Regist 53:29–33
FDA (Food Drug Administration), Guidance for Industry, M3 (R2) (2010) Nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals, January 2010, revision 1
Dyer AR, Burdock GA, Carabin IG, Haas MC, Boyce J, Alsaker R, Read LC (2008) In vitro and in vivo safety studies of a proprietary whey extract. Food Chem Toxicol 46(5):659–665. doi:10.1016/j.fct.2007.12.029
Sathya M, Kokilavani R, Teepa KSA (2012) Acute and subacute toxicity studies of ethanolic extract of Acalypha indica Linn in male wistar albino rats. Asian J Pharm Clin Res 5(Suppl 1):97–100
Bhunia AK, Johnson MC, Ray B, Belden EL (1990) Antigenic property of pediocin AcH produced by Pediococcus acidilactici H. J Appl Bacteriol 69(2):211–215
Akhila JS, Deepa S, Alawar MC (2011) Acute toxicity studies and determination of madian lethal dose. Curr Sci 93(7):917–920
OECD (Organisation for Economic Cooperation Development) (1987) Guidelines for the testing of chemicals OECD 401. Acute oral toxicity. Organisation for Economic Cooperation and Development, Paris
Jozala AF, de Andrade MS, de Arauz LJ, Pessoa AJR, Penna TC (2007) Nisin production utilizing skimmed milk aiming to reduce process cost. Appl Biochem Biotech 137-140(1–12):515–528. doi:10.1007/s12010-007-9076-9
Dabour N, Zihler A, Kheadr E, Lacroix C, Fliss I (2009) In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol 133(3):225–233. doi:10.1016/j.ijfoodmicro.2009.05.005
Gowda S, Desai PB, Hull VV, Math AAK, Vernekar SN, Kulkarni SS (2009) A review on laboratory liver function tests. Pan Afr Med J 3(17)
Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AAK, Vernekar SN (2010) Markers of renal function tests. N Am J Med Sci 2(4):170–173
Eaton DL, Klaassen CD (2001) Principles of toxicology, In; Klassen Cd (ed) Casarett and Doull’s toxicology: the basic science of poisons, 6th edn. McGraw-Hill Medical Publishing Division, p 11–34
Benford D (2000) The acceptable daily intake a tool for ensuring food safety. International Life Sciences Institute (ILSI), Europe. http://www.pac.gr/bcm/uploads/c2000acc_dai.pdf. Accessed 28 Nov 2016
Tsai CC, Liu TH, Chen MH, Tsai CC, Tsen HY (2004) Toxicity evaluation for an Enterococcus faecium strain TM39 in vitro and in vivo. Food Chem Toxicol 42(10):1601–1609. doi:10.1016/j.fct.2004.05.005
Acknowledgements
The authors are thankful to Intas Pharmaceutical Limited (Biopharma Division), Nirma Education and Research Foundation (NERF), Ahmedabad (India) and School of Life Sciences, Sambalpur University, Sambalpur (India), for providing research and infrastructure facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The procedure used for study followed the guidelines and approved by Institutional Animal Ethical Committee (IAEC) (Protocol No. IS/FAC/13-1/038), and experiments were carried out in animal house, Institute of Pharmacy, Nirma University, Ahmedabad, India.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sahoo, T.K., Jena, P.K., Prajapati, B. et al. In Vivo Assessment of Immunogenicity and Toxicity of the Bacteriocin TSU4 in BALB/c Mice. Probiotics & Antimicro. Prot. 9, 345–354 (2017). https://doi.org/10.1007/s12602-016-9249-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9249-3