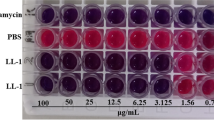
Abstract
Continual occurrence of foodborne outbreaks, along with the increase in antibiotic resistance which burdens clinical treatments, has urged scientists to search for other potential promising antimicrobial agents. Antimicrobial peptides are emerging as one of the potential alternatives. The mode of action of a given AMP is critical and essential for future application; however, it is still not completely known for many of these compounds. Ib-AMP1 is a plant-derived AMP, purified from seeds of Impatiens balsamina and has been shown to exert antibacterial and antifungal activity at the micromolar level. A study had shown that the therapeutic index of Ib-AMP1 against eight human pathogens is 23.5. The objective of the present study was to determine the in vivo mode of action of Ib-AMP1 against Escherichia coli O157:H7. A concentration-dependent effect of Ib-AMP1 on the E. coli O157:H7 cell membrane occurred. Ib-AMP1 treatments resulted in efflux of K+ and ATP, suggesting pores of sufficient size to allow efflux of large molecules. Ib-AMP1 at sublethal concentrations exerts a greater effect at the intracellular level. In contrast, Ib-AMP1 at a lethal concentration permeabilizes cell membranes and may directly or indirectly inhibit intracellular macromolecule synthesis. Collectively, results of this study suggest Ib-AMP1 is bactericidal interfering within outer and inner membrane integrity permitting efflux of ATP and interfering with intracellular biosynthesis of DNA, RNA and protein.





Similar content being viewed by others
Abbreviations
- AMPs:
-
Antimicrobial peptides
- pAMPs:
-
Plant-derived antimicrobial peptides
- SDDW:
-
Sterile de-ionized distilled water
- NPN:
-
N-Phenyl-1-naphthylamine
- DiSC3(5):
-
3,3-Dipropylthiadicarbocyanine iodide
- RT:
-
Room temperature
References
Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM (2011) Foodborne illness acquired in the United States- unspecified agents. Emerg Ingect Dis 17(1):16–22
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM (2011) Foodborne illness acquired in the United States- mojor pathogens. Emerg Infect Dis 17(1):7–15
Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV (1999) Food-related illness and death in the United States. Emerg Infect Dis 5(5):607–625
BC center for disease control (2009) Writing your own food safety plan- the HACCP way-a guide for food service operations. http://www.bccdc.ca/foodhealth/foodguidelines/default.htm. Accessed 7 November 2012
Meng J, Zhao S, Doyle MP, Joseph SW (1998) Antibiotic resistance of Escherichia coli O157:H7 and O157:NM isolated from animals, foods, and humans. J Food Prot 61(11):1511–1514
Musgrove MT, Jones DR, Northcutt JK, Cox NA, Harrison MA, Fedorka-Cray PJ, Ladely SR (2006) Antimicrobial resistance in Salmonella and Escherichia coli isolated from commercial shell eggs. Poult Sci 85(9):1665–1669
Ganz T, Lehrer RI (1998) Antimicrobial peptides of vertebrates. Curr Opin Immunol 10(1):41–44
García-Olmedo F, Molina A, Alamillo JM, Rodríguez-Palenzuéla P (1998) Plant defense peptides. Biopolymers 47(6):479–491
Bulet P, Hetru C, Dimarcq JL, Hoffmann D (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23(4–5):329–344
van’t Hof W, Veerman EC, Helmerhorst EJ, Amerongen AV (2001) Antimicrobial peptides: properties and application. Biol Chem 382(4):597–619
Hammami R, Ben Hamida J, Vergoten G, Fliss I (2009) PhytAMP: a database dedicated to antimicrobial plant peptides. Nucleic Acids Res 37(1):D963–D968
Barbosa Pelegrini P, Del Sarto RP, Silva ON, Franco OL, Grossi-de-Sa MF (2011) Antimicrobial peptides from plants: what they are and how they probably work. Biochem Res Int 2011:250349
Thomma BP, Cammue BP, Thevissen K (2002) Plant defensins. Planta 216(2):193–202
Lay FT, Anderson MA (2005) Defensins: components of the innate immune system in plants. Curr Protein Pept Sci 6(1):85–101
Cândido ES, Porto WF, Amaro DA, Viana JC, Dias SC, Franco OL (2011) Structural and functional insights into plant bactericidal peptides. In: Mendez-Vilas A (ed) Science against microbial pathogens: communicating current research and technological advances, vol. 2. Spain: Formatex Research Center, pp. 951–960. ISBN (13): 978-84-939843-2-8
Tailor RH, Acland DP, Attenborough S, Cammue BP, Evans IJ, Osborn RW, Ray JA, Rees SB, Broekaert WF (1997) A novel family of small cysteine-rich antimicrobial peptides from seed of Impatiens balsamina is derived from a single precursor protein. J Biol Chem 272(39):24480–24487
Wang P, Bang JK, Kim HJ, Kim JK, Kim Y, Shin SY (2009) Antimicrobial specificity and mechanism of action of disulfide-removed linear analogs of the plant-derived Cys-rich antimicrobial peptide Ib-AMP1. Peptides 30(12):2144–2149
Patel SU, Osborn R, Rees S, Thornton JM (1998) Structural studies of Impatiens balsamina antimicrobial protein (Ib-AMP1). Biochemistry 37(4):983–990
Lee DG, Shin SY, Kim DH, Seo MY, Kang JH, Lee Y, Kim KL, Hahm KS (1999) Antifungal mechanism of a cystein-rich antimicrobial peptide, Ib-AMP1, from Impatiens balsamina against Candida albicans. Biotechnol Lett 22(12):1047–1050
Thevissen K, Francois IE, Sijtsma L, van Amerongen A, Schaaper WM, Meloen R, Posthuma-Trumpie T, Broekaert WF, Cammue BP (2005) Antifungal activity of synthetic peptides derived from Impatiens balsamina antimicrobial peptides Ib-AMP1 and Ib-AMP4. Peptides 26(7):1113–1119
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788(8):1687–1692
Matsuzaki K, Sugishita K, Fujii N, Miyajima K (1995) Molecular basis for membrane selectivity of an antimicrobial peptide, Magainin 2. Biochemistry 34(10):3423–3429
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta 1462(1–2):11–28
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial action and resistance. Pharmacol Rev 55(1):27–55
Cudic M, Otvos L Jr (2002) Intracellular targets if antibacterial peptides. Curr Drug Targets 3(2):101–106
Mead PS, Griffin PM (1998) Escherichia coli O157:H7. Lancet 352(9135):1207–1212
Orlov DS, Nguyen T, Lehrer RI (2002) Potassium release, a useful tool for studying antimicrobial peptides. J Microbiol Methods 49(3):325–328
Yasuda K, Ohmizo C, Katsu T (2003) Potassium and tetraphenylphosphonium ion-selective electrodes for monitoring changes in the permeability of bacterial outer and cytoplasmic membranes. J Microbiol Methods 54(1):111–115
Murdock C, Chikindas ML, Matthews KR (2010) The pepsin hydrolysate of bovine lactoferrin causes a collapse of the membrane potential in Escherichia coli O157:H7. Probiotics Antimicro Prot 2(2):112–119
Suzuki M, Yamamoto T, Kawai Y, Inoue N, Yamazaki K (2005) Mode of action of piscicocin CS526 produced by Carnobacterium pisicola CS526. J Appl Microbiol 98:1146–1151
Breeuwer P, Abee T (2004) Assessment of the membrane potential, intracellular pH and respiration of bacterial employing fluorescence techniques. In: Kowalchuck GA, de Brulin FJ, Head IM, Akkermans AD, van Elsas JD (eds) Molecular microbial ecology manual, 2nd edn. Kluwer Academic Publishers, Netherlands, pp 1563–1580
Loh B, Grant C, Hancock RE (1984) Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26(4):546–551
Cotsonas King A, Wu L (2009) Macromolecular synthesis and membrane perturbation assays for mechanisms of action studies of antimicrobial agents. Curr Protoc Pharmacol Chapter 13: 13A.7.1–13A.7.23
Xiong YQ, Bayer AS, Yeaman MR (2002) Inhibition of intracellular macromolecular synthesis in Staphylococcus aureus by thrombin-induced platelet microbicidal protein. J Infect Dis 185(3):348–356
McEntire JC, Carman GM, Montville TJ (2004) Increased ATPase activity is responsible for acid sensitivity of nisin-resistant Listeria monocytogenes ATCC 700302. Appl Environ Microbiol 70(5):2717–2721
Guihard G, Bénédetti H, Besnard M, Letellier L (1993) Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem 268(24):17775–17780
Ultee A, Kets EPW, Smid EJ (1999) Mechanisms of action of carvacrol on the foodborne pathogen Bacillus cereus. Appl Environ Microbiol 65(10):4606–4610
Pol IE, Krommer J, Smid EJ (2002) Bioenergistic consequences of nisin combined with carvacrol towards Bacillus cereus. Innov Food Sci Emerg Technol 3(1):55–61
Hilpert K, McLeod B, Yu J, Elliott MR, Rautenbach M, Ruden S, Burck J, Muhle-Goll C, Ulrich AS, Keller S, Hancock REW (2010) Short cationic antimicrobial peptides interact with ATP. Antimicrob Agents Chemother 54(10):4480–4483
Stephan J, Mailaender EtienneG, Daffé M, Niederweis M (2004) Multidrug resistance of a porin deletion mutant of Mycobacterium smegmatis. Antimicrob Agents Chemother 48(11):4163–4170
Kotera Y, Inoue M, Mitsuhashi S (1990) Activity of KB-5246 against outer membrane mutants of Escherichia coli and Salmonella typhirium. Antimicrob Agents Chemother 34(7):1323–1325
Nikaido K, Nakae T (1979) The outer membrane of gram-negative bacteria. Adv Microb Physiol 20:163–250
Hancock RE, Raffle VJ, Nicas TI (1981) Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 19(5):777–785
Hancock RE, Wong PG (1984) Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother 26(1):48–52
Helander IM, Nurmiaho-Lassila EL, Ahvenainen R, Rhoades J, Roller S (2001) Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int J Food Microbiol 71(2–3):235–244
Alakomi HL, Skyttä E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM (2000) Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 66(5):2001–2005
Leive L (1965) Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun 21(4):290–296
Leive L (1974) The barrier function of gram-negative envelop. Ann N Y Acad Sci 235:109–129
Hukari R, Helander IM, Vaara M (1986) Chain length heterogeneity of lipopolysaccharide released from Salmonella typhimurium by ethylenediaminetetraacetic acid or polycations. Eur J Biochem 154(3):673–676
Acknowledgments
The work was supported by a research grant from the Center for Advanced Food Technology, Rutgers, The State University of New Jersey, New Brunswick, New Jersey.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, WH., Di, R. & Matthews, K.R. Antibacterial Mode of Action of Ib-AMP1 Against Escherichia coli O157:H7. Probiotics & Antimicro. Prot. 5, 131–141 (2013). https://doi.org/10.1007/s12602-013-9127-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-013-9127-1