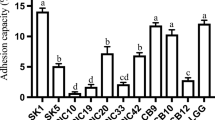
Abstract
In this study, we evaluated the occurrence of antibacterial peptide (ABP)-producing Bacillus spp. in fermented foods. Among 78 isolated cultures, 25 potential ABP-producing stains were selected and differentiated genotypically and phenotypically. The 16S rRNA gene sequence homology, in combination with morphological, physiological and biochemical characteristics, was used for the identification of the isolates. The isolates exhibited inhibitory activity against both Gram-positive and Gram-negative food-borne pathogens. The antibacterial compounds produced by these cultures were proteinaceous in nature, with molecular weight falling in the range of 3–6.5 kDa. The ABP present in the cell-free supernatant of B. subtilis Ec1 and B. licheniformis Me1 exhibited the highest titre of activity (3,400 AU/ml) and wide range of pH (4–10) and temperature (40–100 °C) stability. The strain Ec1 was found to be exhibiting some in vitro probiotic properties, such as acid and bile tolerance, bile salt hydrolase activity and hydrophobicity towards hydrocarbons. The viable counts of Listeria monocytogenes Scott A in pasteurized milk samples containing ABP of Ec1 were lower than those observed in controls without ABP. The ABP-coated packaging films exhibited antimicrobial activity against the pathogens, indicating the application of ABP from Bacillus spp. in antimicrobial packing materials. These observations increase the likelihood of potential use of the isolated Bacillus spp. or their ABP for application in food biopreservation and as probiotics.




Similar content being viewed by others
References
Abriouel H, Franz CMAP, Omar NB, Galvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35:201–232
Baruzzi F, Quintieri L, Morea M, Caputo L (2011) Antimicrobial compounds produced by Bacillus spp. and applications in food. In: Méndez-Vilas (ed) Science against microbial pathogens: communicating current research and technological advances, vol 2. Formatex Research Center, pp 1102–1111, ISBN (13):978-84-939843-2-8
Beaumont M (2002) Flavouring composition prepared by fermentation with Bacillus spp. Int J Food Microbiol 75:189–196
Begley M, Cotter PD, Hill C, Ross RP (2009) Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl Environ Microbiol 75:5451–5460
Bizani D, Morrissy JAC, Dominguez APM, Brandelli A (2008) Inhibition of Listeria monocytogenes in dairy products using the bacteriocin-like peptide cerein 8A. Int J Food Microbiol 121:229–233
Bonade A, Murelli F, Vescovo M, Scolari G (2001) Partial characterization of a bacteriocin produced by Lactobacillus helveticus. Lett Appl Microbiol 33:153–158
Chateau N, Deschamps AM, Hadj-Sassi A (1994) Heterogeneity of bile salts resistance in the Lactobacillus isolates from a probiotic consortium. Lett Appl Microbiol 18:42–44
Cherif A, Ouzari H, Daffonchio D, Cherif H, Ben SK, Hassen A, Jaoua S, Boudabous A (2001) Thuricin 7: a novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett Appl Microbiol 32:43–247
Chou L, Weimer B (1999) Isolation and characterization of acid and bile-tolerant isolates from strains of Lactobacillus acidophilus. J Dairy Sci 82:23–31
Claus D, Berkeley RCW (1986) Genus Bacillus Cohn 1872, 174. In: Sneath PHA, Mair NS, Sharpe ME, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 2. Williams and Wilkins, Baltimore, MD, pp 1105–1139
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71:1–20
Conway PL, Gorbach SL, Goldin BR (1987) Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci 70:1–12
Cosson C, Deschamps AM (1994) Behavior of probiotic strains in the presence of bile and bile salts. Nutr Food Microbiol 12:93–98
Durkee DL (2012) Coming out of the dairy case: new developments in shelf stable probiotic foods. http://www.foodmaster.com. (Date accessed: Feb 2012)
EUCAST (2011) Breakpoint tables for interpretation of MICs and zone diameters. Version 1.3, Jan 2011. Available online: http://www.eucast.org/clinical_breakpoints/
Galvez A, Abriouel H, Lopez RL, Omar NB (2007) Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120:51–70
Gilbert P, Evans DJ, Evans E, Duguid IG, Brown MRW (1991) Surface characteristics and adhesion of Escherichia coli and Staphylococcus epidermidis. J Appl Bacteriol 71:72–77
Gilliland SE, Staley TE, Bush LJ (1984) Importance in bile tolerance of Lactobacillus acidophilus used as a diatery adjunct. J Dairy Sci 67:3045–3051
Halami PM (2008) Production of polyhydroxyalkanoate from starch by the native isolate Bacillus cereus CFR06. World J Microbiol Biotechnol 24:805–812
Han JH (2000) Antimicrobial food packaging. Food Technol 54:56–65
Havenaar R, Huis In’t Veld JMJ (1992) Probiotics: a general view. In: Wood BJB (ed) The Lactic acid bacteria in health and disease, vol 1. Elsevier Applied Science, London, UK, pp 209–224
Hyronimus B, Le Marrec C, Hadj Sassi A, Deschamps A (2000) Acid and bile tolerance of spore-forming lactic acid bacteria. Int J Food Microbiol 61:193–197
Islam VIH, Babu NP, Pandikumar P, Ignacimuthu S (2011) Isolation and characterization of putative probiotic bacterial strain, Bacillus amyloliquefaciens, from North East Himalayan soil based on in vitro and in vivo functional properties. Probiotics Antimicrob Proteins 3:175–185
Jeyaram K, Mohendro SW, Premarani T, Devi AR, Chanu KS, Talukdar NC, Singh MR (2008) Molecular identification of dominant microflora associated with ‘Hawaijar’—a traditional fermented soybean (Glycine max (L.)) food of Manipur, India. Int J Food Microbiol 122:259–268
Kamal K, Eitan BD, Arieh Z, Zeev B (1990) The fate of Bacillus thuringiensis var. israelensis in B. thuringiensis var. israelensis-killed pupae of Aedes aegypti. J Invertebr Pathol 56:312–316
Khalil R, Elbahloul Y, Djadouni F, Omar S (2009) Isolation and partial characterization of a bacteriocin produced by a newly isolated Bacillus megaterium 19 strain. Pak J Nutr 8:242–250
Lee KH, Jun KD, Kim WS, Paik HD (2001) Partial characterization of polyfermenticin SCD, a newly identified bacteriocin of Bacillus polyfermenticus. Lett Appl Microbiol 32:146–151
Lin ASH, Teo AYL, Meng TH (2007) Antimicrobial compounds from Bacillus subtilis for use against animal and human pathogens; US Patent 7247299
Lisboa MP, Bonatto D, Bizani D, Henriques JAP, Brandelli A (2006) Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. Int Microbiol 9:111–118
Martirani L, Varcamonti M, Naclerio G, De Felice M (2002) Purification and partial characterization of bacillocin 490, a novel bacteriocin produced by a thermophilic strain of Bacillus licheniformis. Microb Cell Fact 1:1–5
Mauriello G, De Luca E, La Storia A, Villani F, Ercolini D (2005) Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett Appl Microbiol 41:464–469
McDougall LA, Holzapfel WH, Schillinger U, Feely DE, Rupnow JH (1994) Scanning electron microscopy of target cells and MW determination of a bacteriocin produced by Lactococcus lactis D53. Int J Food Microbiol 24:295–308
Motta AS, Brandelli A (2002) Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol 92:63–71
Muriana PM, Klaenhammer TR (1991) Purification and partial characterization of Lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol 57:114–121
Natrajan N, Sheldon BW (2000) Efficacy of nisin coated polymer films to inactivate Salmonella typhimurium on fresh broiler skin. J Food Prot 63:1189–1196
Nicholson WL, Setlow P (1990) Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM (eds) Molecular biological methods for Bacillus. Wiley, Chichester, pp 391–450
Nithya V, Halami PM (2012) Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann Microbiol. doi:10.1007/s13213-012-0453-4
Oscariz JC, Cintas L, Holo H, Lasa I, Nes IF, Pisabarro AG (2006) Purification and sequencing of cerein 7B, a novel bacteriocin produced by Bacillus cereus Bc7. FEMS Microbiol Lett 254:108–115
Pattnaik P, Kaushika JK, Grover S, Batish VK (2001) Purification and characterization of a bacteriocin-like compound (Lichenin) produced anaerobically by Bacillus licheniformis isolated from water buffalo. J Appl Microbiol 91:636–645
Sanders ME, Morelli L, Tompkins TA (2003) Spore formers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr Rev Food Sci Food Saf 2:101–110
Sarkar PK, Hasenack B, Nout MJR (2002) Diversity and functionality of Bacillus and related genera isolated from spontaneously fermented soybeans (Indian Kinema) and locust bean (African Soumbala). Int J Food Microbiol 77:175–186
Schagger H, von Jagow G (1987) Tricine-SDS PAGE for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schillinger U, Geisen R, Holzapfel WH (1996) Potential of antagonistic micro-organisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol 7:158–164
Schillinger U, Guigas C, Holzapfel WH (2005) In vitro adherence and other properties of lactobacilli used in probiotic yoghurt like products. Int Dairy J 15:1289–1297
Sharma N, Kapoor G, Gautam N, Neopaney B (2009) Characterization of partially purified bacteriocin of Bacillus spp. MTCC 43 isolated from rhizosphere of radish (Raphanus sativus) and its application as a potential food biopreservative. J Sci Ind Res 68:881–886
Sharp RJ, Scawen MD, Atkinson T (1989) Fermentation and downstream processing of Bacillus. In: Harwood CR (ed) Biotechnology handbook: Bacillus. Plenum Press, New York, pp 255–292
Singh TP, Kaur G, Malik RK, Schillinger U, Guigas C, Kapila S (2012) Characterization of intestinal Lactobacillus reuteri strains as potential probiotics. Probiotics Antimicrob Proteins 4:47–58
Slepecky RA, Hemphill HE (1992) The genus Bacillus-nonmedical. In: Balows A, Truper HG, Dworkin M, Schleifer KH, Harder W (eds) The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, vol 2, 2nd edn. Springer, New York, pp 1663–1696
Soomro AH, Masud T, Kiran A (2002) Role of lactic acid bacteria (LAB) in food preservation and human health—a review. Pak J Nutr 1:20–24
Sorokulova IB, Pinchuk IV, Denayrolles M, Osipova IG, Huang JM, Cutting SM, Urdaci MC (2008) The safety of two Bacillus probiotic strains for human use. Dig Dis Sci 53:954–963
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Stein T, Borchert S, Conrad B, Feesche J, Hofemeister B, Hofemeister J, Entian KD (2002) Two different lantibiotic like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J Bacteriol 184:1703–1711
Tamang JP, Tamang N, Thapa S, Dewan S, Tamang B, Yonzan H, Rai AK, Chattri R, Chakarabarty J, Kharel N (2012) Microorganism and ethnic value of ethnic fermented foods and alcoholic beverages from North East India. Indian J Tradit Knowl 11(1):7–25
Westers H, Dorenbos R, van Dijl JM, Kabel J, Flanagan T, Devine KM (2003) Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol Bio Evol 20:2076–2090
Wood BJB (1998) Microbiology of fermented foods, 2nd edn, vol 2. published by Blackie Academic and Professional, an imprint of thomson science, 2-6 boundary row, London SE1 8HN, UK
Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ (2000) A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis 17:3666–3672
Zhang YC, Ronimus RS, Turner N, Zhang Y, Morgan HW (2002) Enumeration of thermophilic Bacillus species in composts and identification with a random amplification polymorphic DNA (RAPD) protocol system. Syst Appl Microbiol 25:618–626
Acknowledgments
The authors acknowledge The Director, CFTRI and The Head, Food Microbiology, CFTRI for providing the facilities. Work was carried out in Institute project (MLP83). NV acknowledges CSIR for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nithya, V., Halami, P.M. Antibacterial Peptides, Probiotic Properties and Biopreservative Efficacy of Native Bacillus Species Isolated from Different Food Sources. Probiotics & Antimicro. Prot. 4, 279–290 (2012). https://doi.org/10.1007/s12602-012-9115-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-012-9115-x