Abstract
We investigated the effect of noxious (pinching) and innocuous (stroking) stimulation of skin on serotonin (5-HT) release in the central nucleus of the amygdala (CeA) in anesthetized rats. 5-HT in the CeA was collected by microdialysis methods. Dialysate output from consecutive 10-min periods was injected into a high-performance liquid chromatograph and 5-HT was measured with an electrochemical detector. Bilateral pinching of the back for 10 min increased 5-HT release significantly; 5-HT release was also increased with stimulation of the forelimb or hindlimb. In contrast, stroking of these areas decreased 5-HT release significantly. Furthermore, simultaneous stroking and pinching produced no change in the 5-HT release. In conclusion, the present study demonstrates that 5-HT release in the CeA is regulated by somatic afferent stimulation in a modality-dependent manner, and that innocuous stimulation can dampen the change in 5-HT release that occurs in response to noxious stimulation.
Similar content being viewed by others
Introduction
Physical stimulation (e.g., cutaneous application of heat, cold, electricity, stroking, etc.) elicits not only motor and autonomic responses but also emotional responses [1–3]. For example, innocuous warmth or touching produces a pleasant feeling and alleviates anxiety, while noxious stimulation produces anxiety and/or fear. Because emotional state can affect the outcome of physical treatments [4], it is important to investigate the mechanisms by which somatic afferent stimulation elicits emotion. In this regard, we have reported in rats that innocuous tactile stimulation (in both anesthetized and awake rats), but not noxious pinching (in anesthetized rats), increases dopamine release in the nucleus accumbens, which is thought to play a key role in motivational and reward processes [5]. Blunted dopamine release in the nucleus accumbens is associated with pathophysiological states, such as depression and anxiety [6–10]. Hence, our prior results suggest that tactile therapies may have the potential to alleviate depression and anxiety via activation of dopaminergic neurons in the nucleus accumbens.
Localized serotonin (5-HT) release in the brain is also responsible for emotional responses. However, serotonergic neurons are widely distributed throughout the brain, and their functions are diverse, with 5-HT being implicated in modulation of sensory input, motor output, and cognition, in addition to emotion [11, 12]. Although release of 5-HT in the somatosensory cortex has been shown to increase following cutaneous stimulation in anesthetized rats [13], the release may not be directly involved in the emotional responses. Furthermore, it has been reported that noxious pinching increases 5-HT release in the amygdala and prefrontal cortex [14], both of which have been implicated robustly in emotional responses. Because this study has been done in awake rats, it is unclear whether these neurophysiological responses are triggered by physical stimulation only, or if they involve psychological factors.
In the present study, we examined 5-HT release in the central nucleus of the amygdala (CeA), a critical structure in anxiety and fear-related behavior [15, 16]. Specifically, we examined how noxious pinching and innocuous stroking affects 5-HT release in the CeA in anesthetized rats to determine whether these physical forms of stimulation alter 5-HT release in the absence of secondarily evoked-emotion or conscious perception. We also investigated the laterality of 5-HT responses in CeA because our prior study demonstrated a laterality effect of stroking on dopamine release in the nucleus accumbens [5].
Materials and methods
All experiments were conducted in accordance with the Japanese Physiological Society Guide for the Care and Use of Laboratory Animals, and the study was approved by the animal ethics committee of the International University of Health and Welfare.
Animals
Experiments were performed on 27 male Wistar rats (270–370 g). The animals were kept in a temperature-controlled room (23 ± 1 °C) that was lit between 08:00 and 20:00 h (Showa Co. Ltd., Tokyo). Commercial rodent chow (Labo-MR stock, Nosan Corporation, Kanagawa) and tap water were provided ad libitum.
Implantation of guide cannula
At 1 or 2 days prior to the experiment, the rats were anesthetized with pentobarbital (50 mg kg−1 i.p.) and implanted stereotaxically with a guide cannula (diameter 0.5 mm; AG-12, Eicom, Kyoto) containing a removable obturator (diameter 0.35 mm; AD-12, Eicom) aimed at the left CeA (in three rats the right CeA was targeted). The placement coordinates (from [17]) were as follows: 2.3 mm posterior to bregma, 4.0 mm lateral to midline, and 6.4 mm below the dura. The guide cannula was secured to the skull with a screw and dental cement. Immediately after surgery, the animals were transferred to individual cages.
General experimental procedures
The experiments were performed under urethane anesthesia (1.1 g kg−1 i.p.). The trachea was intubated for spontaneous breathing. Core temperature was maintained at 37.5 ± 0.1 °C with a heating pad and an infrared lamp (ATB-1100, Nihon-Kohden, Tokyo). Throughout the experiment, depth of anesthesia was assessed routinely by counting respiration rate, and by observing corneal and flexion reflexes.
Microdialysis probe implantation and dialysate sampling
On the morning of the experiment day, a concentric microdialysis probe with a 1-mm membrane (220 µm outer diameter, 50-kDa molecular weight cut-off; A-I-12-01, Eicom) was inserted into the left (or right) CeA via the previously implanted guide cannula. The probe inlet was connected to a Teflon tube (JT-10, Eicom) via a byton tube (JB-30, Eicom) and perfused with modified Ringer’s solution (147 mM Na+, 4 mM K+, and 1.15 mM Ca2+) at a rate of 1 μl min−1. The probe outlet was also connected to a Teflon tube via a byton tube, and the dialysate was collected from the outlet tube for 10 min. Pooled dialysate samples were injected manually into a high-performance chromatograph every 10 min for analysis. The in vitro recovery rate of 5-HT recorded by individual microdialysis probes ranged from 8.4 to 12.0 %. In order to avoid the differences in the recovery rate in each probe, the 5-HT concentration in the dialysate was calculated at 10.0 % recovery.
Measurement of 5-HT
5-HT was measured using a high-performance liquid chromatograph with an electrochemical detector (HPLC-ECD, Eicom). The mobile phase [0.1 M sodium phosphate buffer (pH 6.0), 500 mg l−1 sodium 1-decanesulfonate, 500 mg l−1 EDTA2Na, and 1 % methanol] was pumped at a rate of 0.5 ml min−1. 5-HT was isolated on a reverse-phase column (4.6 ϕ × 30 mm, EICOMPAK PP-ODS, Eicom) and quantitated with a graphite electrode (WE-3G, Eicom) set to a detector potential of +400 mV against a Ag/AgCl reference electrode. The coefficient of variation of this method for a standard solution of 0.06 fmol μl−1 was 0.95 % (n = 8).
Cutaneous stimulation
Noxious mechanical stimulation was produced by pinching, which consisted of applying a surgical clamp bilaterally (force, 3–5 kg) for 10 min to the forelimb (between the shoulder and wrist), back (between the inferior angle of the scapula and the iliac crest) or hindlimb (between the iliac crest and knee joint). During the 10-min stimulation period, a new site was pinched every 2 min to avoid accommodation to the stimulus. Innocuous mechanical stimulation was produced by manual stroking (pressure, 80–100 mmH2O) of the aforementioned areas for 10 min [rate, 4–5 cm s−1; frequency 65–75 strokes min−1 (1.08–1.25 Hz)]. In some experiments, stimulation was applied unilaterally. Each stimulus type was applied to each area once or twice per rat, and data from identical procedures were pooled to produce an averaged response data for each animal.
Probe placement verification
After completion of the experiment, each rat was anesthetized deeply with sodium pentobarbital, and its brain was removed after transcardial perfusion of formalin. Coronal sections (50 μm) were stained with Nissl and examined microscopically to determine the precise location of the microdialysis probe by referring to the atlas of Paxinos and Watson [17]. Placement of the probe was confirmed to be in the CeA for all of the rats used in this study.
Statistical analysis
Data were expressed as mean ± SD. Group comparisons were made with a Student’s t test or analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Probability values of less than 5 % were considered significant.
Results
Effects of bilateral cutaneous stimulation of the back on 5-HT release in the CeA
Basal 5-HT output in the CeA dialysate in the unstimulated condition (n = 6) was 0.84 ± 0.20 fmol 10 μl−1 (i.e., 0.84 ± 0.20 fmol 10 min−1), and sequential samplings of the dialysate were stable over a 60-min unstimulated collecting period (Fig. 1a).
5-HT release in the CeA in response to bilateral cutaneous stimulation of the back. Ordinates: response magnitude is expressed as a percentage (mean ± SD) of the prestimulus control value. Abscissa: 0 indicates the onset of stimulation. Horizontal bar indicates the 10-min stimulus period. a Baseline experiments. b Bilateral pinching of the back. c Bilateral stroking of the back. *p < 0.01 vs. prestimulus control values. n = 6
When pinching was applied to the back bilaterally in the same animals, the 5-HT concentration (prestimulus basal level 0.91 ± 0.32 fmol 10 μl−1) in the CeA dialysate increased significantly (123 ± 11 % of basal value) during the stimulation period. The concentration returned to basal levels during the subsequent 10-min sampling period (10–20 min after the onset of stimulation) (Fig. 1b).
When stroking was applied to the back bilaterally in the same animals, the concentration of 5-HT in the CeA dialysate (basal concentration 0.92 ± 0.27 fmol 10 μl−1) decreased significantly (84 ± 6 % of basal value) during the stimulation period (Fig. 1c). A slight (not statistically significant) decrease in 5-HT secretion was seen 10–30 min after the onset of stimulation.
Effects of cutaneous stimulation of the limbs on 5-HT release in the CeA
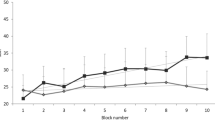
The effects of pinching and stroking of limbs on 5-HT output were examined in a different group of animals (n = 6). Bilateral forelimb and bilateral hindlimb pinching resulted in increases of up to 119 ± 8 % and up to 125 ± 11 % of prestimulus control values, respectively (Fig. 2a). Conversely, bilateral forelimb and bilateral hindlimb stroking resulted in decreases to as low as 83 ± 8 and 86 ± 4 % of prestimulus control values, respectively (Fig. 2b). Pinching and stroking effects on 5-HT release did not differ in relation to which cutaneous area was stimulated.
5-HT release in the CeA in response to pinching (a) and stroking (b) of each tested skin area. Peak responses (values observed during the stimulus period) were compared. Ordinates: response magnitude is expressed as a percentage of the prestimulus control value. *p < 0.01 vs. prestimulus control value. n = 6
Laterality effect of noxious but not innocuous stimulation on 5-HT release
The effects of stimulus laterality on 5-HT release were examined in another cohort of animals (n = 6). When pinching stimulation of the back was applied contralateral (right side) to the site of 5-HT measurement, the concentration of 5-HT in the left CeA dialysate increased significantly (115 ± 3 % of prestimulus control values) during the stimulation period (Fig. 3a). The 5-HT concentration returned to prestimulus levels immediately after cessation of the stimulation (10–20 min after the onset of stimulation). In contrast, when pinching of the back was applied ipsilateral (left side) to the site of 5-HT measurement, the concentration of 5-HT in the left CeA dialysate did not change over a 40-min observation period after the onset of stimulation (Fig. 3a). Similarly, increases of 5-HT release in the right CeA were observed with contralateral (left side), but not ipsilateral (right side), stimulation (data not shown; n = 3).
5-HT release in the CeA in response to pinching (a) and stroking (b) of the back contralateral or ipsilateral to the dialyzed CeA. Triangle stimulation ipsilateral to 5-HT measurement site. Inverted triangle stimulation contralateral to 5-HT measurement site. † p < 0.01 (ipsilateral), *p < 0.01 (contralateral) vs. prestimulus control value. n = 6. See Fig. 1 for other details
When stroking of the back was applied contralateral (right side) to the site of 5-HT measurement, the concentration of 5-HT in the left CeA dialysate decreased (87 ± 4 % of prestimulus control values) during the stimulation period (Fig. 3b). The 5-HT concentration returned to prestimulus control levels immediately after cessation of the stimulation (10–20 min after the onset of stimulation). A similar decrease was observed during the stimulation period when stroking was applied ipsilateral (left side) to the site of 5-HT measurement (86 ± 3 % of prestimulus control values) (Fig. 3b). Decreases in 5-HT release in the right CeA were also observed following both contralateral (left side) and ipsilateral (right side) stimulation (data not shown; n = 3).
Effects of simultaneous pinching and stroking on 5-HT release
Effects of simultaneous noxious pinching and innocuous stroking of the back on 5-HT release were examined in another cohort of anesthetized animals (n = 6). Pinching was applied contralaterally, while stroking was applied ipsilaterally to the site of 5-HT measurement. Pinching of the back contralateral to the dialyzed site increased the concentration of 5-HT in the CeA dialysate significantly (113 ± 3 % of prestimulus control values) during the stimulation period, whereas ipsilateral stroking decreased the concentration of 5-HT in the CeA dialysate significantly (85 ± 4 % of prestimulus control values) (Fig. 4a, b). When both of these stimulation procedures were applied simultaneously, no significant changes in 5-HT release were observed (Fig. 4c).
Changes in 5-HT release in the CeA in response to simultaneous pinching and stroking. Peak responses (values during stimulation period) were compared. a Only pinching applied to the back contralateral to 5-HT measurement. b Only stroking applied to the back ipsilateral to 5-HT measurement. c Both pinching and stroking applied simultaneously to opposite sides of the back. See Fig. 2 for other details. *p < 0.01 vs. prestimulus control values. # p < 0.01 between treatments. n = 6
Discussion
The main finding of the current study was that cutaneous mechanical stimulation produced changes in 5-HT release within the CeA in anesthetized rats, and that the directionality of these changes was dependent upon stimulus modality. That is, innocuous stroking decreased 5-HT release whereas noxious pinching increased 5-HT release. Furthermore, simultaneous stroking and pinching resulted in no change in the 5-HT release in the CeA. Given that 5-HT release in the CeA is associated with anxiety and fear [15, 16], it is conceivable that the present findings provide scientific evidence of how stroking has relieving (anxiolytic) effects on the anxiety or fear induced by noxious stimulation [18, 19].
Handling has been reported to increase release of dopamine in the amygdala, lateral septum, and ventrolateral neostriatum of rats [20–22], whereas pinching has been reported to result in an increased release of 5-HT in the hippocampus, corpus striatum, amygdala, and prefrontal cortex [14]. However, because these prior investigations were conducted with rats that were awake, it was unclear whether the results were due to the pure effects of physical stimulation or to the influence, at least in part, of psychological factors.
The effects of physical stimulation, without contributions from psychological factors, should be examined in anesthetized animals. Few studies have compared the effects of stroking and pinching on neurotransmitter release in anesthetized animals; exceptions include studies that examined the release of acetylcholine, 5-HT, and noradrenaline in the somatosensory cortex [13, 23]. In these studies, acetylcholine release increased in response to both of stroking and pinching, while 5-HT and noradrenaline release increased in response to pinching, but not stroking. Additionally, dopamine release in the nucleus accumbens has been shown to increase in response to stroking, but not pinching [5]. To our knowledge, the present study is the first to demonstrate opposite effects on neurotransmitter release being induced by innocuous versus noxious somatic stimulation. Given that serotonergic neurons originate in the dorsal raphe nucleus (DRN) [24, 25], the present findings suggest that innocuous stroking may inhibit serotonergic neurons in the DRN, whereas noxious pinching may excite them.
The basal released 5-HT levels observed in the CeA in the present study (under anesthesia) were far lower (one-fifth to one-tenth) than those reported for awake rats in previous studies [26–30]. However, direct comparison of the present basal values with those in previous studies is hampered by the use of different experimental conditions, including the use of different microdialysis probes. In this regard, we observed basal values of 5-HT release in the CeA of awake rats in a preliminary study and obtained values that did not differ greatly from those reported in the present study. Changes in CeA 5-HT release in awake animals in response to noxious and innocuous cutaneous stimulation have not reported. However, it was reported previously that 5-HT levels in the CeA during immobilization were 160~200 % of prestimulus values [27]. The greater-magnitude effect observed in the prior immobilization study, relative to the effect observed here in response to pinching, may be due to the combined effects of physical and psychological factors in a conscious state.
The CeA responses to pinching and stroking were similar across the different skin areas examined in this study. This observation fits with prior work showing that CeA neurons have wide receptive fields (e.g., nociceptive neurons in the CeA respond to stimuli applied to the tail, trunk, face, cornea, tongue, and intraoral regions, and all four limbs) [31]. Furthermore, these general 5-HT responses are similar to dopamine release responses in the nucleus accumbens, which have been implicated in the emotion of pleasantness [5]. Conversely, acetylcholine, 5-HT, and noradrenaline release in the primary somatosensory cortex shows stimulus site specificity; the release of these neurotransmitters increased in response to stimulation of the forelimb and hindlimb, but not stimulation of the face and back [13, 23]. Taken together, these results suggest that brain areas related to emotion may be influenced by wide-ranging cutaneous areas.
Release of 5-HT in the CeA in response to pinching showed a laterality effect. Only pinching applied contralateral to the CeA where 5-HT was being measured increased 5-HT release (see Figs. 3a, 5a). Noxious afferent inputs enter mainly lamina I and II of the spinal cord, and it has been shown that 95 % of the projection neurons from lamina I convey their signals to the contralateral LPBN (lateral parabrachial nucleus) [32], which has projections to the CeA [33, 34]. Given that there are also CeA-DRN projections [35], it may be that noxious dermal stimulation follows the pathway from the spinal cord via the LPBN to the CeA, and then back to the DRN, where serotonergic neurons to the CeA originate. Additionally, both the CGRP (calcitonin gene-related peptide) neurons projecting from the LPBN to the CeA and the CGRP-receptive neurons in the CeA have been reported to be involved in freezing [36]. Hence, it could be that the 5-HT response to pinching in the CeA involves CGRP neurotransmission.
Diagram summarizing the present results. a Pinching of the skin increases 5-HT release in the contralateral CeA. b Stroking of the skin decreases 5-HT release in both the ipsilateral and contralateral CeA. c Increased 5-HT release in the contralateral CeA in response to pinching was abolished with simultaneous application of stroking. CeA the central nucleus of the amygdala, 5-HT serotonin, plus excitatory influence, minus inhibitory influence
On the contrary, 5-HT responses to innocuous stroking were not lateralized. Stimulation had similar decreasing effects on 5-HT release in the CeA regardless of whether it was applied contralaterally or ipsilaterally (see Figs. 3b, 5b). Stroking excites mainly Group II afferent fibers, which transmit signals to the thalamic nuclei contralateral to the side of the spinal cord into which they entered via both the dorsal column pathway and the spinothalamic pathway, resulting in contralateral dominance. Some (~5 %) of the neurons in the dorsal column nucleus project to the ipsilateral thalamus [37], which projects to the lateral amygdala, and then to the CeA [38, 39]. However, this ipsilateral transmission pathway seems insufficient to account for the decreased 5-HT release observed in this study in response to ipsilateral stroking, which was similar in magnitude to that produced by contralateral stroking. If so, there must be some unknown pathways that convey stroking-triggered signals from the periphery to the ipsilateral CeA.
When pinching and stroking were applied simultaneously on opposite sides of the body, release of 5-HT in the CeA showed no changes (see Figs. 4c, 5c). These results suggest that stroking had the effect of inhibiting the increased 5-HT release observed when only pinching was applied, or vice versa. Given that release of 5-HT in the CeA is associated with the generation of anxiety and fear [15, 16], we assume that pleasant somatosensory stimulation (e.g., stroking) may weaken the anxiety and fear caused by noxious stimulation, apparently by dampening the increased release of 5-HT release in the CeA.
In humans, the right amygdala is activated predominantly in response to frightful or painful stimulation [40, 41]. Neuronal responses in the right CeA are larger than those in the left CeA in monoarthritic rats [42]. However, 5-HT release in response to pinching and stroking did not differ between the right and left CeA in the present study. In accordance, it was shown that the neuronal responses of the CeA to noxious and innocuous stimulations are not different in the right and left CeA under normal conditions in rats [42].
Limitations
The present experiment was conducted with anesthetized animals to preclude contributions of psychological responses to somatic stimulations. However, the use of anesthesia is associated with some limitations. Firstly, because the animals were not awake, we could not observe emotional behavioral responses to stimulation, such as freezing, which are considered to be produced consequent to changes in 5-HT release in the CeA. Furthermore, loss of inhibition from the medial prefrontal cortex to the CeA [43–45] in the anesthetized brain might alter 5-HT release in the CeA in response to somatic stimulation relative to that which would normally occur in awake animals.
Conclusions
The present findings demonstrate, for the first time, that innocuous stroking decreases, while noxious pinching increases 5-HT release in the CeA. The increased 5-HT release observed in response to pinching was abolished when there was simultaneous application of innocuous stroking. These results may provide scientific evidence for the relieving effects of soothing somatosensory stimulation (e.g., stroking) on anxiety and fear evoked by pain and stressors.
References
Villemure C, Bushnell MC (2009) Mood influences supraspinal pain processing separately from attention. J Neurosci 29:705–715
Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC (2002) Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 5:900–904
Wiech K, Tracey I (2009) The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 47:987–994
Sinyor D, Amato P, Kaloupek DG, Becker R, Goldenberg M, Coopersmith H (1986) Post-stroke depression: relationships to functional impairment, coping strategies, and rehabilitation outcome. Stroke 17:1102–1107
Maruyama K, Shimoju R, Ohkubo M, Maruyama H, Kurosawa M (2012) Tactile skin stimulation increases dopamine release in the nucleus accumbens in rats. J Physiol Sci 62:259–266
Shirayama Y, Chaki S (2006) Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4:277–291
Yadid G, Friedman A (2008) Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res 172:265–286
Shimamoto A, Debold JF, Holly EN, Miczek KA (2011) Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology 218:271–279
Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ (2011) An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery 69:1281–1290
Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, Sidor MM, Birnbaum SG, Graham A, Neve RL, Gordon E, Ozburn AR, Goldberg MS, Han MH, Cooper DC, McClung CA (2011) Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology 36:1478–1488
Cools R, Roberts AC, Robbins TW (2008) Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12:31–40
Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM (2008) Serotonin: a review. J Vet Pharmacol Ther 31:187–199
Kurosawa M, Sato A, Zhou W (1993) Cutaneous noxious mechanical sensory stimulation increases extracellular release of noradrenaline and serotonin in the cerebral cortex in anesthetized rats. Biog Amines 10:27–37
Rueter LE, Jacobs BL (1996) A microdialysis examination of serotonin release in the rat forebrain induced by behavioral/environmental manipulations. Brain Res 739:57–69
Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ (2006) Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience 141:1047–1055
Li Q, Luo T, Jiang X, Wang J (2012) Anxiolytic effects of 5-HT(1)A receptors and anxiogenic effects of 5-HT(2)C receptors in the amygdala of mice. Neuropharmacology 62:474–484
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Field TM (1998) Massage therapy effects. Am Psychol 53:1270–1281
Field T (2014) Massage therapy research review. Complement Ther Clin Pract 20:224–229
Adams F, Schwarting RK, Boix F, Huston JP (1991) Lateralized changes in behavior and striatal dopamine release following unilateral tactile stimulation of the perioral region: a microdialysis study. Brain Res 553:318–322
Adams BW, Moghaddam B (2000) Tactile stimulation activates dopamine release in the lateral septum. Brain Res 858:177–180
Inglis FM, Moghaddam B (1999) Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem 72:1088–1094
Kurosawa M, Sato A, Sato Y (1992) Cutaneous mechanical sensory stimulation increases extracellular acetylcholine release in cerebral cortex in anesthetized rats. Neurochem Int 21:423–427
Petrov T, Krukoff TL, Jhamandas JH (1994) Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res 277:289–295
Li YQ, Jia HG, Rao ZR, Shi JW (1990) Serotonin-, substance P- or leucine-enkephalin-containing neurons in the midbrain periaqueductal gray and nucleus raphe dorsalis send projection fibers to the central amygdaloid nucleus in the rat. Neurosci Lett 120:124–127
Palazzo E, Marabese I, Soukupova M, Luongo L, Boccella S, Giordano C, de Novellis V, Rossi F, Maione S (2011) Metabotropic glutamate receptor subtype 8 in the amygdala modulates thermal threshold, neurotransmitter release, and rostral ventromedial medulla cell activity in inflammatory pain. J Neurosci 31:4687–4697
Mo B, Feng N, Renner K, Forster G (2008) Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull 76:493–498
Li H, Scholl JL, Tu W, Hassell JE, Watt MJ, Forster GL, Renner KJ (2014) Serotonergic responses to stress are enhanced in the central amygdala and inhibited in the ventral hippocampus during amphetamine withdrawal. Eur J Neurosci 40:3684–3692
Smriga M, Kameishi M, Uneyama H, Torii K (2002) Dietary l-lysine deficiency increases stress-induced anxiety and fecal excretion in rats. J Nutr 132:3744–3746
Macedo CE, Martinez RC, de Souza Silva MA, Brandao ML (2005) Increases in extracellular levels of 5-HT and dopamine in the basolateral, but not in the central, nucleus of amygdala induced by aversive stimulation of the inferior colliculus. Eur J Neurosci 21:1131–1138
Bernard JF, Huang GF, Besson JM (1992) Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68:551–569
Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836
Bernard JF, Peschanski M, Besson JM (1989) A possible spino (trigemino)-ponto-amygdaloid pathway for pain. Neurosci Lett 100:83–88
Bernard JF, Besson JM (1990) The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63:473–490
Retson TA, Van Bockstaele EJ (2013) Coordinate regulation of noradrenergic and serotonergic brain regions by amygdalar neurons. J Chem Neuroanat 52:9–19
Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD (2015) Elucidating an affective pain circuit that creates a threat memory. Cell 162:363–374
Wree A, Itzev DE, Schmitt O, Usunoff KG (2005) Neurons in the dorsal column nuclei of the rat emit a moderate projection to the ipsilateral ventrobasal thalamus. Anat Embryol (Berl) 210:155–162
Turner BH, Herkenham M (1991) Thalamoamygdaloid projections in the rat: a test of the amygdala’s role in sensory processing. J Comp Neurol 313:295–325
Price JL (2003) Comparative aspects of amygdala connectivity. Ann N Y Acad Sci 985:50–58
Ahs F, Pissiota A, Michelgard A, Frans O, Furmark T, Appel L, Fredrikson M (2009) Disentangling the web of fear: amygdala reactivity and functional connectivity in spider and snake phobia. Psychiatry Res 172:103–108
Lu CL, Wu YT, Yeh TC, Chen LF, Chang FY, Lee SD, Ho LT, Hsieh JC (2004) Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil 16:575–587
Ji G, Neugebauer V (2009) Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 102:2253–2264
Pape HC, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90:419–463
Amir A, Amano T, Pare D (2011) Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105:3054–3066
Pinard CR, Mascagni F, McDonald AJ (2012) Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205:112–124
Acknowledgments
We thank Mr. Yuki Masuya, Eicom Co. Ltd., for his skillful technical assistance in measuring 5-HT, and Ms. Rie Yamaguchi, Tokyo University of Agriculture and Technology, for her technical assistance in our histological examination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Tokunaga, R., Shimoju, R., Takagi, N. et al. Serotonin release in the central nucleus of the amygdala in response to noxious and innocuous cutaneous stimulation in anesthetized rats. J Physiol Sci 66, 307–314 (2016). https://doi.org/10.1007/s12576-015-0426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-015-0426-z