Abstract
Anandamide (AEA), one of endocannabinoids, has been reported to exhibit a cardioprotective ability to limit the damage produced by ischemia–reperfusion injury. AEA reportedly enhanced heat shock protein 72 (HSP72) and HSP25 expression in lungs to protect against lung inflammation. This study tested the hypothesis that intravenously injected AEA would induce HSP72 in the heart and thus render cardioprotection against ischemia–reperfusion injury in rats. Cardiac expression of HSPs was quantitatively evaluated in rats by Western blot analysis. That intravenously injected AEA 1 mg/kg in vivo induced expression of HSP72, which peaked at 24 h after administration. The enhancement of HSP72 by AEA was blocked by cannabinoid 2 (CB2) receptor antagonist AM630, but not cannabinoid 1 (CB1) receptor antagonist AM251. Therefore, the rats were induced with a 30-min coronary occlusion followed by a 120-min reperfusion in vivo at 24 h after administration of drugs or vehicle, and then the infarct size was measured. AEA reduced myocardial infarct size compared to control group. Pretreatment with AM630 but not AM251 abolished the infarct size-limiting effect of AEA. Further study demonstrated pretreatment with phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin, Akt inhibitor MK-2206 and AM630 attenuated phosphorylation of Akt and AEA-induced HSP72 expression. The results suggest that AEA is cardioprotective against ischemia–reperfusion insult through its induction of HSP72, which might be mediated by the PI3K/Akt signaling pathway. These effects were mediated by CB2 but not CB1 receptors.
Similar content being viewed by others
Introduction
The heat shock proteins (HSPs) are an important family of endogenous protective proteins that increase in response to a wide variety of stresses, such as heat shock, hypoxia, hydrogen peroxide, inflammation and ischemia [1]. Among the various sizes of HSPs, HSP72 is reportedly involved predominantly in cardioprotection; several studies have demonstrated that whole-body hyperthermia 24 h before the onset of myocardial ischemia is protective against ischemia–reperfusion injury and is associated with proportional induction of HSP72 expression [2]. Furthermore, recent studies revealed that both the myogenic cells and hearts of transgenic mice overexpressing HSP72 protein are resistant to ischemic injury [3].
Anandamide (AEA) is one of the endocannabinoids, which are involved in many physiological and pathophysiological processes, such as neurobehavior, gastrointestinal function, stress and anxiety, and cardiovascular functions [4]. At least two types of cannabinoid (CB) receptors, the CB1and CB2, have been found, and these two receptors are widespread in many tissues, including cardiac myocyte [5]. It has been shown that AEA protects the heart from arrhythmias induced by ischemia–reperfusion [6]. Similarly, AEA can limit the damage induced by ischemia–reperfusion in rat isolated hearts [7]. Is the mechanism of protection related to enhancement of HSP72? Recently, AEA administration was reported to enhance HSP72 and HSP25 expression in lungs [8]. However, there is no report on whether AEA can enhance HSP72 expression in hearts.
The present experiments were designed to test the hypothesis that AEA, as a cardioprotective substance [9], can enhance the expression of cytoprotective HSP72, a protein highly inducible in hearts following exposure to a variety of stressors [10], and provide protection against ischemia–reperfusion injury.
Materials and methods
Animals and drugs
Experiments were carried out in adult male Sprague-Dawley rats (weighing 230–280 g) obtained from the Experimental Animal Center of Hebei Province. This study was performed conforming to Guide for the Care and Use of Laboratory Animals described by Directive 2010/63/EU of the European Parliament. Animal work was approved by the Ethics Committee for Animal Experiments of Hebei Medical University, in compliance with the NIH, and carried out in compliance with China government guidelines.
AEA (Cayman Chemical Corp., USA) was injected intravenously (i.v.) into experimental rats at a dose of 1 mg/kg, based on previously published methods [11]. The rats were anesthetized with ketamine and xylazine (80 and 4 mg/kg i.p., respectively) before cannulation surgery. The right femoral vein was cannulated for administration of drugs. The stock solution of AEA was in 96 % ethanol. Prior to use, dilutions were made with saline in order to minimize the effect of the solvent and to obtain the final 9.6 % concentration of ethanol containing the proper dose of the drug. The drugs were administered at intervals of at least 30 min to avoid any accumulation effect. Injections were completed within 2 s. All injections had a volume of 0.2 ml and were followed by a flush of 0.1 ml saline. Ethanol (9.6 %) in saline was used as a control for AEA.
To determine the receptor subtypes that mediated the effect of AEA, rats received CB1 receptor antagonist AM251 (1 mg/kg, Cayman Chemical Corp., USA) or CB2 receptor antagonist AM630 (1 mg/kg, Cayman Chemical Corp., USA) intravenously together with AEA (1 mg/kg). To examine the time-dependent expression of HSP72, rats were killed by deep anesthesia with pentobarbital (150 mg/kg i.v.) 6, 12, 24 and 48 h after administration of AEA or vehicle. The heart was rapidly removed and frozen in liquid nitrogen.
To determine the involvement of the PI3K/Akt pathway that mediated the effect of AEA, rats received the PI3K inhibitor wortmannin (15 μg/kg, Sigma Corp., USA) or Akt inhibitor MK-2206 [12] (300 μg/kg, Selleck Chemistry, USA) intravenously together with AEA (1 mg/kg).
Western blotting
Western blotting was performed as previously described [13]. The frozen heart preparations were homogenized with SDS sample buffer, centrifuged and boiled. The total protein concentration of the myocardium was quantified by the Bradford method. The preparations were diluted in dissociation buffer. An equal amount of total protein in each fraction was conducted on 8.5 % SDS-PAGE and transferred electrophoretically to a polyvinylidine difluoride membrane. After transferral and blocking with 0.5 % nonfat milk, the membranes were incubated with antibodies. Membranes were assessed for the presence of HSP72 using primary antibodies: mouse monoclonal against HSP72 (1:100, Stressgen, Stressgen Bioreagents Corp., USA); for the presence of Akt and phospho-Akt using antibodies: rabbit multiclonal Akt and phospho-Akt (1:1000, Cell Signaling Technology, USA). In the next step membranes were incubated with secondary horseradish peroxidase linked antibodies: goat antimouse or mouse antirabbit (1:1000, BioRad, BioRad Laboratories, Inc., USA). Protein bands were visualized with peroxidase substrate kit Vector SG (Vector Laboratories Inc., USA). Such prepared membranes were captured, and the optical density of stained bands was quantified using the Scion Image Program (Scion Corp., USA).
Determination of myocardial infarct size
Twenty-four hours after administration of drugs or vehicle, rats were anesthetized with pentobarbital (150 mg/kg, i.p.), and the body temperature was maintained at 37.0 ± 0.5 °C. Animals were ventilated with a rodent ventilator (HX-300S, Chengdu TME Technology Co., Ltd., China) at 60 to 70 breaths per minute with tidal volume of about 15 ml/kg. The electrocardiogram (ECG) in lead II together with the blood pressure of the carotid artery was continuously monitored and recorded using a data acquisition system (PowerLab/8 s, AD Instrument, Australia). Left thoracotomy was performed in the third or fourth intercostals space, and the pericardium was opened to expose the heart. A 5/0 silk suture was passed around the left descending artery (LDA). After stabilization of cardiac function for 15 min, myocardial ischemia was produced by ligating the LDA, and reperfusion was produced by loosening the ligation [14]. The classical ischemic sign following coronary arterial occlusion was indicated by a significant ST segment elevation on the ECG immediately after LDA ligation together with a slight blood pressure reduction (Fig. 1).
The coronary artery was occluded for 30 min, followed by 120-min reperfusion; 1000 U of sodium heparin was given intravenously before coronary artery occlusion. At the end of reperfusion, after the rat LDAs were ligated completely, the aorta was also ligated, and 2 % Evans blue (1 ml) was injected to the heart via the left free ventricular wall to delineate the in vivo area at risk; then, the heart was removed quickly and frozen. After removal of the atrium and right ventricular wall, the left ventricular wall was sectioned into 2-mm transverse sections from the apex to base (5 slices/heart). Following defrosting, the slices were incubated at 37 °C with 1 % triphenyltetrazolium chloride (TTC) in phosphate buffer (pH 7.4) for 15 min, fixed in 10 % formaldehyde solution and photographed with a digital camera (Cannon, Japanese) to distinguish clearly red-stained viable tissue and unstained necrotic tissue. Normal myocardium stained by Evans blue and TTC looked blue, ischemic myocardium that was not infarcted stained by TTC looked red, and infarct myocardium unstained by either Evans blue or TTC looked pale. The area at risk included a red and a pale area. The different zones were determined using an image processing system (JIE DA-108, Jiangsu, China). The area at risk and left ventricular infarct zone were expressed as percentage of ventricle surface (area at risk/ventricle surface) and area at risk (left ventricular infarct zone/area at risk), respectively.
Statistics
Data were expressed as mean ± SEM. The differences of the parameters between prior and posterior to drug application were analyzed by paired Student’s t test. Differences between groups were evaluated by one-way ANOVA followed by Dunnet’s post hoc test. Statistical significance was accepted at P < 0.05.
Result
Cardiac expression of HSP72
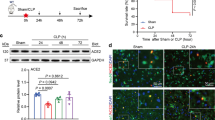
HSP72 in the AEA group was observed from 6 h and peaked at 24 h after AEA administration, but the expression level at 48 h after administration decreased compared with that at 24 h (Fig. 2a). No time-dependent change in the expression of HSP72 was observed in the control group (Fig. 2b). Pretreatment with AEA for 24 h resulted in a high content of HSP72 in myocardium, which was blocked by the CB2 receptor antagonist AM630 but not the CB1 receptor antagonist AM251 (Fig. 2c, d). However, AM630 or AM251 alone had no effect on HSP72 expression.
Expression of heat shock protein 72 (HSP72) after administration of anandamide (AEA) analyzed by Western blot. a Representative bands of HSP72 at baseline (BL), 6, 12, 24 and 48 h after administration of AEA (1 mg/kg). b Plots of relative density of HSP72 protein to BL against time after administration of AEA (AEA group) and vehicle (control group). Representative bands of HSP72 protein (c) and bars of relative density of HSP72 protein (d) at 24 h derived from control, experimental AEA-treated rats, CB1 receptor antagonist AM251-treated rats, AM251- and AEA-treated rats, CB2 receptor antagonist AM630-treated rats, and AM630- and AEA-treated rats. Data are relative density compared to that in the control group (n = 6). Data are mean ± SEM. *P < 0.05 versus control group
Infarct size
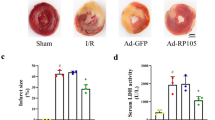
The infarct size in AEA group hearts at 120 min after reperfusion was 24.6 ± 3.7 %, much smaller than 46.7 ± 7.6 % in control rat hearts (P < 0.05). There was no significant difference in the area at risk between the two groups. CB1 receptor antagonist AM251 or CB2 receptor antagonist AM630 alone had no significant effect on the infarct size. However, pretreatment with AM630 but not AM251 abolished the reducing effects of AEA on the infarct size (Fig. 3).
Effects of anandamide (AEA) on myocardial infarct size induced by ischemia–reperfusion. a Representative TTC staining of control hearts, AEA-treated hearts, and AM630- and AEA-treated hearts after ischemia–reperfusion. Infarct size as a percent of area at risk (b) and area at risk as a percent of left ventricle (c) in control, AEA-treated rats, CB1 receptor antagonist AM251-treated rats, AM251- and AEA-treated rats, CB2 receptor antagonist AM630-treated rats, and AM630- and AEA-treated rats. * P < 0.05 versus control group. (n = 6)
PI3K/Akt signaling pathway is involved in AEA-induced HSP72 expression
Phospho-Akt and total Akt were observed before and 1 and 24 h after AEA administration. A robust increase in phospho-Akt was observed 1 h after AEA treatment, which was inhibited by pretreatment with AM630, PI3K inhibitor wortmannin and Akt inhibitor MK-2206, respectively (Fig. 4).
Expression of phospho-Akt (p-Akt) after administration of AEA analyzed by Western blot. a, b Representative bands of p-Akt (a) and bars of relative density of p-Akt (b) at 0 (BL), 1 and 24 h after administration of AEA (1 mg/kg). Representative bands of p-Akt (c) and bars of relative density of p-Akt (d) at 1 h derived from control, experimental AEA-treated rats, AM630- and AEA-treated rats, wortmannin- and AEA-treated rats, MK-2206- and AEA-treated rats. Data are relative density compared to that in the control group (n = 6). Data are mean ± SEM. *P < 0.05 versus control group or baseline
A high content of HSP72 in myocardium was observed 24 h after AEA treatment, which was also blocked by pretreatment with AM630 (Fig. 2c, d), wortmannin and MK-2206 (Fig. 5).
Effects of phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin and Akt inhibitor MK-2206 on HSP72 expression induced by AEA. Representative bands of HSP72 protein (a) and bars of relative density of HSP72 protein (b) at 24 h derived from control, experimental AEA-treated rats, wortmannin- and AEA-treated rats, MK-2206- and AEA-treated rats. Data are relative density compared to that in the control group (n = 6). Data are mean ± SEM. *P < 0.05 versus control group
Discussion
The major findings in this study are that intravenous injection of 1 mg/kg AEA evoked a significant increase in HSP72 expression through the PI3K/Akt signaling pathway in the hearts of rats and that the infarct size of hearts treated with AEA were reduced when suffering ischemia–reperfusion. These effects were mediated by CB2 but not CB1 receptors.
It is reported that AEA can limit the damage induced by ischemia–reperfusion in rat isolated hearts [7]. In our study, AEA also reduced the infarct size in hearts suffering ischemia–reperfusion. Therefore, without doubt, AEA can protect the heart from ischemia–reperfusion injury. HSP72, a cytosolic AEA-binding protein, uses a nonvesicular mechanism to transport AEA intracellularly [15]. During cell stress responses, transportation of the AEA-HSP72 complex could decrease the amount of free HSP72 in the cytosol, while inducing HSP72 expression to obtain intracellular homeostasis. Some authors believe that the pool of free (unbound) cytosolic HSPs is very small and that the further reduction in HSP levels is a signal to induce HSP expression in response to proteotoxic stress [16]. Recent experiments described that AEA administration enhanced HSP72 and HSP25 expression in lungs [8]. Pasquariello et al. [17] also proved that AEA administration induces the overexpression of BiP, an important member of the HSP72 family, in cultured human neuroblastoma cells. Therefore, these results are similar to our finding that AEA increased HSP72 expression in the hearts of rats.
We determined the receptor subtypes responsible for the effect of AEA on HSP72 and the infarct size of hearts suffered ischemia–reperfusion. Two types of cannabinoid receptors, CB1 and CB2, have been cloned [18] and widely expressed in the cardiovascular system, such as blood vessels and cardiac tissue [19]. AEA is a natural constituent of the plasma membrane and considered to be a CB1 and CB2 receptor agonist because it exhibits pharmacological activities comparable to cannabinoids [20]. In this study, we found that blockade of CB2 receptors with the selective antagonist AM630 completely blocked the elevation of AEA-induced HSP72. Also, AM630 abolished the protective effects of AEA on hearts during ischemia–reperfusion. However, the CB1 receptor antagonist AM251 failed to change the effects of AEA on HSP72 and myocardial infarct size during ischemia–reperfusion in hearts. The results indicated that the increase of AEA-induced HSP72 expression and protective effects of AEA against ischemia–reperfusion injury were mediated by the CB2 receptor but not the CB1 receptor. Many studies have emphasized the role of CB2 receptors in cardioprotection. It has been shown that blockade of CB2 receptors eliminates the cardiac protective effect of endocannabioinds in rat isolated hearts exposed to low-flow ischemia and reperfusion [21]. To further strengthen the cardioprotective role of CB2 receptor, a recent study demonstrated that a single dose of the CB2 receptor agonist JWH-133 reduced infarct size [22]. Selective CB2 receptor agonists have antiinflammatory effects in various other models of ischemic–reperfusion injury [23]. These results are consistent with our finding that CB2 receptors are involved in cardioprotection.
Both the myogenic cells and hearts of transgenic mice found to overexpress HSP72 protein are resistant to ischemic injury [3]; in other words, increased HSP72 levels per se provide protection against ischemia-related insult. The above data showed two phenomena: (1) AEA caused elevation of HSP72, and (2) AEA caused cardiac protection. These two phenomena seemed parallel and consistent, but whether or not HSP72 “per se” contributes to the AEA-induced cardiac protection is not known. In our study, blockade of CB2 receptors completely blocked the elevation of AEA-induced HSP72 and moreover abolished the protective effects of AEA during ischemia–reperfusion. That is, the elevation of AEA-induced HSP72 by the CB2 receptor inhibits the ischemia–reperfusion-induced cardiac infarction. Taking these data into account, HSP72 per se is indeed “involved and responsible” in the AEA-induced protection from ischemia–reperfusion-induced cardiac damage.
Further mechanisms of HSP72 induction by AEA were examined. It was reported that phosphatidylinositol 3-kinase (PI3K)-dependent activation of Akt was demonstrated to be essential for the expression of cardiac HSP72 induced by hyperthermia [24]. That stimulation of the CB2 receptor by endocannabinoids has a neuroprotective effect that is achieved through PI3K/Akt signaling [25] was also reported. Is the PI3K/Akt signaling pathway involved in the elevation of AEA-induced HSP72? In our study, AEA could increase phospho-Akt expression; pretreatment with the PI3K inhibitor wortmannin and Akt inhibitor MK-2206 both inhibited the elevation of AEA-induced phospho-Akt. Furthermore, pretreatment with wortmannin and MK-2206 also inhibited the elevation of AEA-induced HSP72. Therefore, we presume that AEA active Akt functions in a PI3K dependent manner, and AEA increased HSP72 though the PI3K/Akt signaling pathway. In addition, these effects were all mediated by the CB2 receptor.
The present experiments are the first to describe the influence of intravenously administered endocannabinoid AEA on HSP expression in hearts. We conclude that AEA administration enhanced HSP72 expression through the PI3K/Akt signaling pathway in hearts. Therefore, AEA-HSP interactions could be involved in mechanisms protecting hearts against ischemia–reperfusion injury. These effects were mediated by CB2 but not CB1 receptors. However, the underlying mechanisms need further investigation.
References
Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132
Walker DM, Pasini E, Kucukoglu S, Marber MS, Iliodromitis E, Ferrari R, Yellon DM (1993) Heat stress limits infarct size in the isolated perfused rabbit heart. Cardiovasc Res 27:962–967
Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL (1996) Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation 94:1408–1411
Hiley CR (2009) Endocannabinoids and the heart. J Cardiovasc Pharmacol 53:267–276
Pacher P, Hasko G (2008) Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 153:252–262
Krylatov AV, Uzhachenko RV, Maslov LN, Ugdyzhekova DS, Bernatskaia NA, Pertwee R, Stefano GB, Makriyannis A (2002) anandamide and r-(+)-methanandamide prevent development of ischemic and reperfusion arrhythmia in rats by stimulation of cb2-receptors. Eksp Klin Farmakol 65:6–9
Underdown NJ, Hiley CR, Ford WR (2005) Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br J Pharmacol 146:809–816
Kopczynska B, Sulejczak D, Welniak-Kaminska M, Gietka A, Grieb P (2012) Anandamide enhances expression of heat shock proteins hsp70 and hsp25 in rat lungs. Eur J Pharmacol 668:257–263
Durst R, Lotan C (2011) The potential for clinical use of cannabinoids in treatment of cardiovascular diseases. Cardiovasc Ther 29:17–22
Latchman DS (2001) Heat shock proteins and cardiac protection. Cardiovasc Res 51:637–646
Kopczynska B (2007) The contribution of vr1 and cb1 receptors and the role of the afferent vagal pathway in modelling of cardio-respiratory effects of anandamide in rats. Life Sci 80:1738–1745
Signorello MG, Giacobbe E, Passalacqua M, Leoncini G (2012) The anandamide effect on no/cgmp pathway in human platelets. J Cell Biochem 112:924–932
Maloyan A, Palmon A, Horowitz M (1999) Heat acclimation increases the basal hsp72 level and alters its production dynamics during heat stress. Am J Physiol 276:R1506–R1515
Johnston KM, MacLeod BA, Walker MJ (1983) Responses to ligation of a coronary artery in conscious rats and the actions of antiarrhythmics. Can J Physiol Pharmacol 61:1340–1353
Oddi S, Fezza F, Pasquariello N, D’Agostino A, Catanzaro G, De Simone C, Rapino C, Finazzi-Agro A, Maccarrone M (2009) Molecular identification of albumin and hsp70 as cytosolic anandamide-binding proteins. Chem Biol 16:624–632
Morimoto RI (2008) Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev 22:1427–1438
Pasquariello N, Catanzaro G, Marzano V, Amadio D, Barcaroli D, Oddi S, Federici G, Urbani A, Finazzi Agro A, Maccarrone M (2009) Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J Biol Chem 284:29413–29426
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65
Pertwee RG (1997) Pharmacology of cannabinoid cb1 and cb2 receptors. Pharmacol Ther 74:129–180
Felder CC, Glass M (1998) Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol Toxicol 38:179–200
Lepicier P, Bouchard JF, Lagneux C, Lamontagne D (2003) Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol 139:805–815
Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S (2009) Cb(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46:612–620
Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P (2007) Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21:1788–1800
Shinohara T, Takahashi N, Ooie T, Hara M, Shigematsu S, Nakagawa M, Yonemochi H, Saikawa T, Yoshimatsu H (2006) Phosphatidylinositol 3-kinase-dependent activation of akt, an essential signal for hyperthermia-induced heat-shock protein 72, is attenuated in streptozotocin-induced diabetic heart. Diabetes 55:1307–1315
Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M (2009) Selective cb2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci 29:4564–4570
Acknowledgments
This work was supported by the National Natural Science Funds of China (no. 31100823), Hebei Medical University Fund (no. 30900166), Hebei Provincial Health Bureau Scientific Research Funds (no. 20110039), and Hebei provincial universities outstanding youth science funds (no. Y2012022).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, Q., Shi, M. & Li, B. Anandamide enhances expression of heat shock protein 72 to protect against ischemia–reperfusion injury in rat heart. J Physiol Sci 63, 47–53 (2013). https://doi.org/10.1007/s12576-012-0228-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-012-0228-5