Abstract
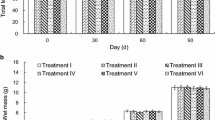
Calcein (CAL) and alizarin red S (ARS) at concentrations of 50–200 and 150–300 mg/l, respectively, were used for immersion marking juvenile grass carp Ctenopharyngodon idellus. With the exception of non-lateral line scales from the 150 mg/l ARS treatment and lateral line scales from the 150, 200 mg/l ARS treatments, immersion for 24 h produced detectable marks in sagittae, lateral line and non-lateral line scales, and fin rays (dorsal, pectoral, ventral, anal, and caudal) at 100 days post-marking. Detectable fluorescent marks in sagittae were readily observed at concentrations of 150–200 mg/l CAL or 200–300 mg/l ARS. Marks were poorly visible in all non-lateral line and lateral line scales from ARS-treated groups. Fluorescent marks were readily detected in non-lateral line and lateral line scales at 150–200 mg/l CAL, and in fin rays at 100–200 mg/l CAL or 150–300 mg/l ARS. In particular, optimal marks were observed at comparatively high concentrations investigated in sagittae (300 mg/l ARS) and fin rays (100–200 mg/l CAL or 250–300 mg/l ARS). There was no significant difference on the survival or growth of marked fish compared to controls throughout the experiment (P > 0.05).







Similar content being viewed by others
References
Zhong Y, Power G (1997) Fisheries in China: progress, problems, and prospects. Can J Fish Aquat Sci 54(1):224–238
Jagdish M, Rana SVS, Agarwal VP (1995) Efficacy of grass carp (Ctenopharyngodon idella) in weed control and its growth in Karna Lake (Haryana). J Inland Fish Soc India 27:49–55
Leslie AJJ, Van Dyke JM, Hestand RS III, Thompson BZ (1987) Management of aquatic plants in multi-use lakes with grass carp (Ctenopharyngodon idella). Lake Reserv Manag 3:266–276
Irons KS, Sass GG, McClelland MA, Stafford JD (2007) Reduced condition factor of two nativefish species coincident with invasion of non-native Asian carps in the Illinois River, USA. Is this evidence for competition and reduced fitness? J Fish Biol 71(sd):258–273
Li YK, Chen Y, Song B, Olson D, Yu N, Chen LQ (2009) Ecosystem structure and functioning of Lake Taihu (China) and the impacts of fishing. Fish Res 95(2):309–324
Mchich R, Charouki N, Auger P, Raïssi N, Ettahiri O (2006) Optimal spatial distribution of the fishing effort in a multi fishing zone model. Ecol Model 197(3–4):274–280
Zhang G, Cao W, Chen Y (1997) Effects of fish stocking on lake ecosystems in China. Acta Hydrobiol Sin 21(3):271–280
Jia PQ, Zhang WB, Liu QG (2013) Lake fisheries in China: challenges and opportunities. Fish Res 140:66–72
MAFBC M.o.A.a.F.B.o.C. (2013) 2012 China Fishery Statistical Yearbook. China Agriculture Press, Peking (in Chinese)
Zhang M, Xie CX, Hansson LA, Hu WM, Che JP (2012) Trophic level changes of fishery catches in Lake Chaohu, Anhui Province, China: trends and causes. Fish Res 131–133:15–20
Lü HJ, Zhang XM, Fu M, Xi D, Gao TX (2014) Use of tetracycline hydrochloride and alizarin complexone for immersion marking black rockfish, Sebastes schlegelii. Chin J Oceanol Limnol 32(4):810–820
Barker JM, McKaye KR (2004) Immersion marking of juvenile midas Cichlids with oxytetracycline. N Am J Fish Manag 24(1):262–269
Taylor MD, Fielder DS, Suthers IM (2005) Batch marking of otoliths and fin spines to assess the stock enhancement of Argyrosomus japonicus. J Fish Biol 66(4):1149–1162
Lv HJ, Zhang XM, Zhang PD, Li WT, Miao ZQ (2011) The implement of plastic oval tags for mark-recapture in juvenile Japanese flounder, Paralichthys olivaceus, on the northeast coast of Shandong Province, China. Afr J Biotechnol 10(61):13263–13277
Hagen P, Munk K, Van Alen B, White B (1995) Thermal mark technology for inseason fisheries management: a case study. Alaska Fish Res Bull 2(2):143–155
Volk EC, Schroder SL, Grimm JJ (1999) Otolith thermal marking. Fish Res 43(1):205–219
Tsukamoto K (1985) Mass-marking of ayu eggs and larvae by tetracycline-tagging of otoliths. Nippon Suisan Gakkaishi 51(6):903–911
Tsukamoto K (1988) Otlith tagging of ayu embryo with fluorescent substances. Nippon Suisan Gakkaishi 54(8):1289–1295
Liu Q, Zhang XM, Zhang PD, Nwafili SA (2009) The use of alizarin red S and alizarin complexone for immersion marking Japanese flounder Paralichthys olivaceus (T.). Fish Res 98(1):67–74
Gelsleichter J, Cortés E, Manire CA, Hueter RE, Musick JA (1997) Use of calcein as a fluorescent marker for elasmobranch vertebral cartilage. Trans Am Fish Soc 126(5):862–865
Lagardère F, Thibaudeau K, Anras MLB (2000) Feasibility of otolith markings in large juvenile turbot, Scophthalmus maximus, using immersion in alizarin-red S solutions. ICES J Mar Sci 57(4):1175–1181
Monaghan JP Jr (1993) Comparison of calcein and tetracycline as chemical markers in summer flounder. Trans Am Fish Soc 122(2):298–301
Honeyfield DC, Ostrowski CS, Fletcher JW, Mohler JW (2006) Dietary calcein marking of brook trout, Atlantic salmon, yellow perch, and coho salmon scales. N Am J Fish Manag 26(2):431–437
Crook DA, O’Mahony D, Gillanders BM, Munro AR, Sanger AC (2007) Production of external fluorescent marks on Golden Perch fingerlings through osmotic induction marking with Alizarin Red S. N Am J Fish Manag 27:670–675
Crook DA, O’Mahony D, Sanger AC, Munro AR, Gillanders BM (2009) Development and evaluation of methods for osmotic induction marking of Golden Perch Macquaria ambigua with Calcein and Alizarin Red S. N Am J Fish Manag 29:279–287
Lü HJ, Zhang XM, Xi D, Gao TX (2014) Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii juveniles. Chin J Oceanol Limnol 32(1):88–98
Baer J, Rösch R (2008) Mass-marking of brown trout (Salmo trutta L.) larvae by alizarin: method and evaluation of stocking. J Appl Ichthyol 24(1):44–49
Brown ML, Powell JL, Lucchesi DO (2002) In-transit oxytetracycline marking, nonlethal mark detection, and tissue residue depletion in yellow perch. N Am J Fish Manag 22(1):236–242
Oliveira K (1996) Field validation of annular growth rings in the American eel, Anguilla rostrata, using tetracycline-marked otoliths. Fish Bull 94(1):186–189
Leips J, Baril CT, Rodd FH, Reznick DN, Bashey F, Visser GJ, Travis J (2001) The suitability of calcein to mark poeciliid fish and a new method of detection. Trans Am Fish Soc 130(3):501–507
Eckmann R (2003) Alizarin marking of whitefish, Coregonus lavaretus otoliths during egg incubation. Fish Manag Ecol 10(4):233–239
Walt BVD, Faragher RA (2003) Otolith marking of rainbow trout fry by immersion in low concentrations of alizarin complexone. N Am J Fish Manag 23(1):141–148
Beckman DW, Schulz RG (1996) A simple method for marking fish otoliths with alizarin compounds. Trans Am Fish Soc 125(1):146–149
Tsukamoto K, Kuwada H, Hirokawa J, Oya M, Sekiya S, Fujimoto H, Imaizumi K (1989) Size-dependent mortality of red sea bream, Pagrus major, juveniles released with fluorescent otolith-tags in News Bay, Japan. J Fish Biol 35(Supplement A):59–69
Yamashita Y, Nagahora S, Yamada H, Kitagawa D (1994) Effects of release size on survival and growth of Japanese flounder Paralichtys olivaceus in coastal waters off Iwate Prefecture, northeastern Japan. Mar Ecol Prog Ser 105:269–276
Ibáñez AL, Rodríguez-Canto A, Cortés-Martínez J, García-Calderón JL (2013) Evaluation of marking efficiency of different alizarin red S concentrations on body fish structures in Oreochromis niloticus (Perciformes: Cichlidae) juveniles. Int J Trop Biol Conserv 61(1):193–201
Bashey F (2004) A comparison of the suitability of alizarin red S and calcein for inducing a nonlethally detectable mark in juvenile guppies. Trans Am Fish Soc 133(6):1516–1523
Skov C, Grønkjær P, Nielsen C (2001) Marking pike fry otoliths with alizarin complexone and strontium: an evaluation of methods. J Fish Biol 59(3):745–750
Mohler JW (2003) Producing fluorescent marks on Atlantic salmon fin rays and scales with calcein via osmotic induction. N Am J Fish Manag 23(4):1108–1113
Purcell SW, Blockmans BF, Nash WJ (2006) Efficacy of chemical markers and physical tags for large-scale release of an exploited holothurians. J Exp Mar Biol Ecol 334(2):283–293
Simon J, Dörner H (2005) Marking the European eel with oxytetracycline, alizarin red and coded wire tags: an evaluation of methods. J Fish Biol 67(5):1486–1491
Frenkel V, Kindschi G, Zohar Y (2002) Noninvasive, mass marking of fish by immersion in calcein: evaluation of fish size and ultrasound exposure on mark endurance. Aquaculture 214(1–4):169–183
Acknowledgments
This work was supported by ther National Natural Science Foundation of China (No. 31400396) and the Fundamental Research Funds for the Central Universities (XDJK2014C019 and SWU113056). We wish to express our thanks to Prof. Weizhi Yao, Shengqi Su, and Zhengli Wu for their suggestions on the manuscript and participation in many aspects of this study. We also thank the three anonymous reviewers for their valuable and constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lü, H., Chen, H., Fu, M. et al. Experimental evaluation of calcein and alizarin red S for immersion marking grass carp Ctenopharyngodon idellus . Fish Sci 81, 653–662 (2015). https://doi.org/10.1007/s12562-015-0884-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0884-5