Abstract
Most reef-building corals in tropical and subtropical areas symbiose with microalgae from the genus Symbiodinium (dinoflagellate) and depend on the photosynthate produced by the microalgae. The majority of corals acquire Symbiodinium from the surrounding environment through horizontal transfer, but the molecular mechanisms involved in the acquisition of Symbiodinium remain unknown. It has been hypothesized that carbohydrate-binding proteins, or lectins, of the host coral recognize cell surface carbohydrates of Symbiodinium in the process of acquiring symbionts. Thus, we examined the molecular mechanisms involving lectins and carbohydrates using model organism Acropora tenuis, a common reef-building coral, and Symbiodinium culture strains. Juvenile polyps acquire more cells of Symbiodinium strain NBRC102920 at 72–96 h of metamorphosis induction than in any other period. Glycosidase treatment of Symbiodinium inhibited the acquisition of Symbiodinium by juvenile coral polyps. The presence of carbohydrates D-galactose, N-acetyl-D-galactosamine, and N-acetyl-D-glucosamine at 10 mM also tended to decrease Symbiodinium acquisition. We isolated two N-acetyl-D-galactosamine binding lectins with apparent molecular masses of 14.6 and 29.0 kDa from A. tenuis, and de novo sequencing and cDNA cloning showed that the 29.0 kDa protein is Tachylectin-2-like lectin (AtTL-2). The anti-Tachylectin-2 antibody is suggested to bind specifically to AtTL-2. The antibody also inhibited binding of AtTL-2 to N-acetyl-D-galactosamine-resin and the acquisition of Symbiodinium by juvenile A. tenuis polyps. Based on these results, AtTL-2 is likely involved in the process of Symbiodinium acquisition.
Similar content being viewed by others
Introduction
Reef-building corals thrive in tropical and subtropical areas and grow in symbiosis with dinoflagellates in the genus Symbiodinium. Symbiodinium have been found in numerous hosts, including a jellyfish, a clam, and a sea slug [1]. Since these dinoflagellates have very similar morphologies, Symbiodinium were classified into phylogenetic clades A through I based on analyses of ribosomal DNA (rDNA) and chloroplast 23S rDNA [2–5]. Symbiodinium strains isolated and cultured from hosts exhibited diurnal changes in morphology, changing from a flagellated motile form to a non-motile coccoid form [6]. The morphology of the coccoid cells is similar to that of Symbiodinium found within the host. The symbiosis between corals and Symbiodinium is essential for the survival of coral, as more than 90 % of the symbiont photosynthate is used by the coral [7]. Bleached corals, which have either lost their Symbiodinium, or which have Symbiodinium that are losing pigments, eventually die [8, 9].
Corals acquire Symbiodinium from the surrounding environment (horizontal transmission) or directly from the parent (vertical transmission); about 85 % of corals acquire Symbiodinium by horizontal transmission [10]. The molecular mechanisms involved in the acquisition of Symbiodinium remain unknown, but some cues for their acquisition have been identified. Symbiodinium modified by digestive enzymes, such as glycosidase and proteinase, or lectin treatments, showed reduced acquisition rates by hosts Aiptasia pulchella and Fungia scutaria [11, 12]. The results of these studies suggest that carbohydrates on the Symbiodinium cell surface play an important role in symbiont acquisition by the host.
Lectins, which are carbohydrate-binding proteins that are widely distributed in organisms from viruses to vertebrates [13], are thought to be involved in non-self recognition, and the participation of lectin in symbiosis has been reported for organisms, such as legumes, sponges, and nematodes [14–16]. It is, therefore, plausible that coral lectins also participate in the acquisition of Symbiodinium. Given the considerable variety of lectins in corals [17–21], it is not clear whether coral lectins play a role in symbiosis.
Interestingly, the galactose-binding lectin SLL-2 purified from coral Sinularia lochmodes [22–24] and CecL purified from coral Ctenactis echinata [25] can arrest some Symbiodinium strains in the coccoid form. Moreover, the lectin gene Pdc-Lectin in coral Pocillopora domicornis was down-regulated 6 days before a bleaching event [26], and a C-type lectin, Millectin, in coral Acropora millepora bound to bacterial pathogens and Symbiodinium [27]. Although these reports suggest that the lectin is involved in the maintenance of symbiosis between Symbiodinium and its coral host, a coral lectin specifically involved in the acquisition of Symbiodinium has not been reported to date.
The coral, A. tenuis has been used as a model organism in previous coral-algal symbiosis studies based on its ability to acquire several Symbiodinium strains [28]. The planula larvae of A. tenuis live for more than 1 month [29, 30], and they can be chemically induced to metamorphose into juvenile polyps [31]. Therefore, in the present study, we sought to establish a model system for Symbiodinium acquisition by A. tenuis polyps, and to identify the lectin(s) involved in the acquisition of symbiotic algae.
Materials and methods
Materials
Larvae of A. tenuis that were either naturally spawned from 2008 to 2011 on Aka Island or Sesoko Island, Okinawa or artificially spawned using a modification of Hayashibara’s method [32] on 20 May 2013 on Ishigaki Island, Okinawa, were collected and maintained in artificial sea water (ASW) MARINE ART SF-1 (Osaka Yakken, Osaka, Japan) at 25 °C. Larvae were grown to the juvenile polyp stage and used for Symbiodinium acquisition experiments. Additionally, some of A. tenuis colonies collected at Ishigaki Island and Sesoko Island, Okinawa were kept at −20 °C until further extraction.
Symbiodinium strains CCMP1633 (clade B), CCMP2467 (clade A type A1), and CCMP2556 (clade D type D1–4) were purchased from the National Center for Marine Algae and Microbiota (East Boothbay, Maine, USA). CS-156 (clade F) and CS-161 (clade A type A3) were purchased from the Commonwealth Scientific and Industrial Research Organisation (Tasmania, Australia). NBRC102920 (clade A type A3) was purchased from the National Institute of Technology and Evaluation (Tokyo, Japan). GTP-A6-Sy (clade A type A2 relative) and AJIS2-C2 (clade A type A1) were originally isolated by Yamashita and Koike [33]. All Symbiodinium strains were cultured in IMK medium for marine microalgae (Wako Pure Chemical Industry, Osaka, Japan) at 25 °C with light at 80 μmol photon m−2 s−1 (12:12 h light:dark cycle).
All reagents not otherwise specified were purchased from Wako Pure Chemical Industry.
Optimization of the conditions for Symbiodinium acquisition by metamorphosed juvenile polyps
Planula larvae and juvenile polyps acquire Symbiodinium, but naturally metamorphosed polyps sometimes do not harbor any Symbiodinium [34]. Because of the ease of observation, we used them for the acquisition experiment. Since Symbiodinium acquisition activity may change during the period after metamorphosis induction in planula larvae, Symbiodinium acquisition activity was examined over 6 days after metamorphosis. Ten planula larvae of A. tenuis were placed individually in the wells of eight-well chambered coverglass (Nunc, Rochester, NY, USA), incubated in 100 μl of ASW with 1.0 μM Hydra-derived neuropeptide, Hym-248 (EPLPIGLW-amide), at 25 °C for 24 h to induce larval settlement and metamorphosis [30], and each chamber was replenished with 400 μl of ASW. After 0, 24, 48, 72, 96, and 120 h, a group of juvenile polyps (n = 5–9) were added with 500 μl of ASW containing 1,000 cells of Symbiodinium strain NBRC102920 having a motility percentage greater than 50 %. The individuals in each treatment were incubated for an additional 24 h at 25 °C with light at 80 μmol photon m−2 s−1 (12:12 h light:dark cycle). A series of optical sections (20 µl thickness) of juvenile polyps were recorded by confocal microscopy (LSM510Meta, Carl Zeiss, Oberkochen, Germany) with in vivo chlorophyll a fluorescence (excitation, 480 nm; emission, 680 nm). The number of Symbiodinium cells within the juvenile polyps was manually counted using these images.
Selection of a Symbiodinium strain suitable for the acquisition by juvenile polyps
Planula larvae of A. tenuis were metamorphosed to juvenile polyps using Hym-248 as described above. At 72 h after the addition of Hym-248, 500 μl of ASW containing 2,500 cells of one of the Symbiodinium strains, having a motile percentage greater than 50 %, was added, and the chambers were incubated at 25 °C for 24 h. The number of Symbiodinium cells within each juvenile polyp was counted as described above except that the detector gain was adjusted for each Symbiodibium strain.
Inhibition of Symbiodinium acquisition due to glycosidase treatment of Symbiodinium
Symbiodinium cells were pretreated with two glycosidases to examine the effect of surface glycan on Symbiodinium acquisition. To 1,000 cells of Symbiodinium strain NBRC102920 in 0.5 ml ASW, 10 μg of glycosidase mix from Turbo cornutus (Seikagaku Corp., Tokyo, Japan), which randomly digests non-reducing terminal oligosaccharides or 1 U of Glycopeptidase F (Roche Diagnosis K. K., Tokyo, Japan), which digests N-glycans of glycoproteins, was added [22], and the mixture was incubated for 4 h and centrifuged at 3,000×g for 5 min. The supernatant was removed, and the pellet was washed with ASW and incubated for 24 h to recover motile cells. Following the method above, 500 cells of glycosidase-treated Symbiodinium cells were added to juvenile polyps, incubated for 24 h, and acquisition was analyzed.
Inhibition of Symbiodinium acquisition due to carbohydrates
The inhibitive effect on Symbiodinium acquisition of the presence of carbohydrates, which are known inhibitors of lectins, was examined. Juvenile polyps at 72 h after the addition of Hym-248 were incubated in ASW at 25 °C for 1 h with the following carbohydrates at a concentration of 10 mM: L-fucose (Fuc), D-galactose (Gal), N-acetyl-D-galactosamine (GalNAc), N-acetyl-D-glucosamine (GlcNAc), N-acetyl-D-neuraminic acid (NANA), and sucrose (Suc). Then, 2,500 cells of Symbiodinium strain NBRC102920 were added to the juvenile polyps and incubated at 25 °C for 6 h. The numbers of Symbiodinium cells within the juvenile polyps were counted using images taken by confocal microscopy.
Preparation of A. tenuis crude extract
A portion of each A. tenuis colony was crushed, mixed with five volumes of extraction buffer (150 mM NaCl, 50 mM Tris–HCl, pH 8.5), and centrifuged at 21,500×g at 4 °C for 10 min. The supernatant was combined with one-fifth weight of polyvinylpolypyrrolidone, which had been washed with ultrapure water and dried, and the mixture was incubated at 4 °C for 30 min with mixing. After centrifugation at 21,500×g at 4 °C for 1 min, the supernatant was collected and stored at −20 °C for further analysis.
Separation of the GalNAc-binding lectin
GalNAc-Sepharose 6B resin was prepared as previously described [24]. Then, 0.1 ml of GalNAc-Sepharose 6B resin was put into a 1.5 ml tube and washed three times with 1 ml of extraction buffer. The pelleted resin was combined with 0.2 ml of crude extract of A. tenuis tissue and incubated at 4 °C overnight with rotation using a Mini Disk Rotor BC-710I (BIO CRAFT, Tokyo, Japan). The tube was centrifuged at 380×g and 4 °C for 1 min, and the supernatant was removed. The resin was washed three times with ten volumes of the extraction buffer supplemented with 100 μl of 0.2 M GalNAc, and then incubated at 4 °C for 30 min. After centrifugation at 380×g and 4 °C for 1 min, lectin was obtained as the supernatant.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
The crude extract of A. tenuis and each of the fractions obtained by affinity separation were treated by reduction-alkylation as follows: 10 μl of sample buffer (20 % glycerol, 4 % SDS, 125 mM Tris–HCl, pH 6.8, 0.01 % bromophenol blue) and 4 μl of 1 M dithiothreitol were added to 10 μl of each protein sample, and incubated at 65 °C for 20 min. After cooling to room temperature, 2.5 μl of 1 M iodoacetamide was added to the sample solution, and the mixture was incubated at room temperature for 20 min in the dark. Finally, the sample solution was neutralized by the addition of 3 μl of 1 M Tris–HCl, pH 9.5. SDS-PAGE was performed on 15 % polyacrylamide gels, according to Laemmli [35]. SDS-PAGE standard Broad Range (Bio Rad Laboratories, Hercules, CA, USA) was used as the protein standards. The gel was stained by zinc reverse staining [36].
De novo sequencing of GalNAc-binding proteins by MALDI-TOF/MS
GalNAc-binding proteins were separated by SDS-PAGE and stained using Oriole Fluorescent Gel Stain (Bio-Rad Laboratories). The protein bands were excised and destained three times with 0.1 ml of 50 % methanol containing 16 mM Tris–HCl, pH 8.0. The gel was washed with 0.1 ml acetonitrile and then dried for 20 min at room temperature. After the addition of 10 μl of 5 μg ml−1 trypsin (proteomics grade, Sigma–Aldrich, St. Louis, MO, USA) in 50 mM NH4HCO3, the mixture was incubated at 37 °C overnight. Digested peptides were extracted with 10 μl of 49 % acetonitrile containing 0.1 % TFA by sonication for 10 min in ice water, and the supernatant was dried using the Centrifugal Evaporator (CE1D, Hitachi Koki, Tokyo, Japan).
Guanidination and sulfonation were performed according to Beardsley et al. [37] and Chen et al. [38] with some modifications. The tube containing dried peptides was added with 12 μl of O-MIU mix (1.5 μl of 1 mg μl−1 O-methylisourea hemisulfate, 5.5 μl of 7 M NH4OH, 5.0 μl of water) and incubated at 65 °C for 15 min. The peptides were bound to ZipTip C18 pipette tips (Millipore, Bedford, MA, USA) and incubated with 10 μl of 10 mg ml−1 4-sulfophenyl isothiocyanate in 20 mM NaHCO3 at 55 °C for 30 min. Modified peptides were eluted from the ZipTip C18 using 80 % acetonitrile containing 0.1 % TFA and mixed with 10 mg ml−1 MassPREP MALDI Matrix CHCA (Waters, Milford, MA, USA) in 50 % acetonitrile containing 0.1 % TFA. The mixture was deposited on an MTP 384 target plate (ground steel T F, Bruker Daltonics, Bremen, Germany) and dried. Mass spectrometry was performed using a MALDI-TOF/MS Autoflex III (Bruker Daltonics). The mass spectra were acquired in the reflectron mode. The standard peptides used are angiotensin II (Sigma–Aldrich) and insulin B chain (Sigma–Aldrich). Among the obtained peaks, sulfonated peaks were analyzed by MS/MS analysis using LIFT. The mass spectra were analyzed by open source mass spectrometry tool mMass [39].
Sequence similarity search
The obtained partial amino acid sequences were compared with sequences in a publicly available putative protein database of A. digitifera (Acropora digitifera Genome, Ver 1.1; http://marinegenomics.oist.jp/genomes/viewer?project_id=3¤t_assembly_version=oist_v1.1) by FASTS analysis [40]. The similarity search of the obtained proteins was performed using BLASTP [41].
cDNA cloning of Tachylectin-2-like cDNA
Total RNA was extracted from fresh adult A. tenuis using RNAiso PLUS (TaKaRa Bio, Otsu, Japan). mRNA was purified from the total RNA using a Poly (A) Purist MAG Kit (Ambion, Austin, TX, USA). Based on the amino acid sequence alignment of Tachylectin-2 homologue of corals (Fig. 1), the PCR primers were designed from the homologous regions at residues 64–69 (5′-AAATTCCTGTTCTTYCACCC-3′) and residues 232–238 (5′-CAACCAGTYATCACYCCRT-3′). Using these primers, PCR was performed with Advantage 2 Polymerase Mix (Clontech, Palo Alto, CA, USA) with the following cycle condition: (94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min) × 2 cycles, (94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min) × 2 cycles, (94 °C for 30 s, 56 °C for 30 s, 72 °C for 1 min) × 2 cycles, (94 °C for 30 s, 54 °C for 30 s, 72 °C for 1 min) × 2 cycles, and (94 °C for 30 s, 52 °C for 30 s, 72 °C for 1 min) × 20 cycles. The amplified PCR products were directly sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA).
Comparison of peptide sequences derived from Acropora tenuis 29.0 kDa protein, Tachylectin-2 and three related species of genus Acropora and AtTL-2. Black boxes and gray boxes indicate identical and similar amino acids, respectively. J indicates I or L and X indicates an undetermined amino acid. Thin lines indicates the regions used to design the PCR primers. Thick lines indicate regions used to design the gene-specific primers. An arrow indicates the starting position of a mature protein of Tachylectin-2. Each doublet indicates a repetitive region. Sequences used were A. tenuis, a 29.0 kDa protein with an identical amino acid sequence determined by de novo sequencing, A. digitifera Tachylectin-2 (adi_v1.08085) with the same deduced amino acid sequence of A. millepora Tachylectin-2 (GenBank accession no. EZ038328), and A. tenuis Tachylectin-2 (http://www.bio.utexas.edu/research/matz_lab/matzlab/Data.html, isotig20551 and isotig22486), AtTL-2 (DDBJ accession no. AB972924) and Tachylectin-2 from Tachypleus tridentatus (UniProtKB accession no. Q27084)
Two gene-specific primers were designed based on the obtained sequences (forward primer, 5′-ATCGCCCACGCCACCCTTATTG-3′ and reverse primer, 5′-AGTTGGGGCTGATCGCTTGTAGA-3′), and RACE was performed with a SMARTer RACE cDNA Amplification Kit (Clontech) according to manufacturer protocol. The cycle conditions were as follows: (94 °C for 30 s, 64 °C for 30 s, 72 °C for 1 min) × 5 cycles, (94 °C for 30 s, 62 °C for 30 s, 72 °C for 1 min) × 5 cycles, and (94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min) × 20 cycles. The RACE products were cloned into pCR4-TOPO (Invitrogen, Carlsbad, CA, USA) and sequenced with a DNA sequencer (ABI3130, Applied Biosystems). The determined nucleotide sequence of AtTL-2 cDNA was deposited to DDBJ with Accession no. AB972924.
Amino acid sequence alignment of Tachylectin-2 homologues
The deduced amino acid sequence of AtTL-2 was aligned with that of Tachylectin-2 from Acopora and Tachypleus tridentatus using ClustalW [42], and this alignment was colored with BoxShade 3.21 (BoxShade Server; http://www.ch.embnet.org/software/BOX_form.html).
Dot blotting using the anti-Tachylectin-2 antibody
The crude extract of A. tenuis and each of the fractions obtained by affinity separation (30 μl) were applied to a polyvinylidene difluoride membrane Fluorotrans W (Pall, Port Washington, NY, USA) using a Bio-Dot apparatus (Bio-Rad Laboratories). Binding of antibodies was performed with a SNAP i.d. (Millipore) according to the manufacturer's protocol. Briefly, the membrane was blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) and incubated with 0.1 μg ml−1 of anti-Tachylectin-2 antibodies raised from Tachylectin-2 (courtesy of Dr. Kawabata) [43] in Can Get Signal Solution 1 (TOYOBO, Osaka, Japan) for 10 min. After washing with PBS-T (0.1 % Tween-20 in phosphate-buffered saline), the membrane was incubated with 1.0 μg ml−1 of horseradish peroxidase (HRP)-labeled anti-rabbit IgG antibodies (Wako Pure Chemical Industry) in Can Get Signal Solution 2 (TOYOBO) for 10 min and then washed with PBS-T. Detection was performed using Luminata Forte Western HRP Substrate (Millipore).
Inhibition of carbohydrate binding activity of AtTL-2 by anti-Tachylectin-2 antibodies
One microliter of anti-Tachylectin-2 antibodies (0.1 mg ml−1) or the extraction buffer was added to 60 µl of the crude extract of A. tenuis (0.37 mg ml−1) and the mixture was incubated at 4 °C for 1 h. Using the mixture, lectins contained were partially purified according to the separation method for the GalNAc-binding lectin.
Inhibition of Symbiodinium acquisition by anti-Tachylectin-2 antibodies
The involvement of AtTL-2 for Symbiodinium acquisition was examined. Rabbit IgG or anti-Tachylectin-2 antibodies (1.5 μg ml−1) was added in ASW to juvenile polyps at 72 h after the addition of Hym-248 and incubated at 25 °C for 1 h before the addition of 2,500 cells of Symbiodinium strain NBRC102920 followed by an additional incubation at 25 °C for 6 h. The numbers of Symbiodinium cells within the juvenile polyps were counted on images obtained using confocal microscopy.
Statistical analysis
Results of Symbiodinium acquisition tests were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s or Dunnett’s multiple comparison test with GraphPad Prism 4 for Macintosh (GraphPad software, CA, USA).
Results
Establishment of an experimental model for Symbiodinium acquisition by juvenile polyps
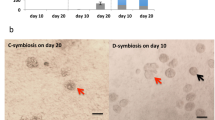
The number of Symbiodinium cells acquired by polyps after Hym-248 treatment was greatest at 72 h (11.7 ± 2.0 cells/polyp) and 96 h (9.3 ± 1.0 cells/polyp) and decreased thereafter (Fig. 2a). Acquisition activity was greatest for NBRC102920 (52.2 ± 14.2 cells/polyp) and CCMP2556 (38.8 ± 26.4 cells/polyp), followed by CCMP2467 (9.4 ± 3.4 cells/polyp), AJIS2-C2 (8.2 ± 3.9 cells/polyp), CS-161 (3.1 ± 2.3 cells/polyp), GTP-A6-Sy (0.3 ± 0.5 cells/polyp), CS-156 (0.3 ± 0.5 cells/polyp), and CCMP1633 (0 cells/polyp) (Fig. 2b). The number of Symbiodinium cells in juvenile polyps differed among Symbiodinium belonging to these clades: NBRC102920 (clade A), CCMP2556 (clade D), CS-156 (clade F), and CCMP1633 (clade B). Taken together, the optimal acquisition model was determined as the introduction of Symbiodinium strain NBRC102920 to juvenile polyps at 72 h after treatment with Hym-248, and this procedure was used in subsequent acquisition experiments.
Optimal experimental conditions for Symbiodinium acquisition by juvenile polyps. a Juvenile polyps treated with Hym-248 were incubated for 24, 48, 72, 96, 120, and 144 h, and then were incubated in the ASW with Symbiodinium strain NBRC102920 for 24 h. Symbiodinium cells within juvenile polyps were counted using Symbiodinium chlorophyll a fluorescence with confocal microscopy. Values are mean ± SD (n = 5–9). Different letters indicate significant differences between incubated times after Hym-248 treatment (P < 0.05, Tukey’s multiple range test). b Juvenile polyps were incubated with each Symbiodinium strain for 24 h. Symbiodinium cells within juvenile polyps were counted. Values are mean ± SD (n = 5). Different letters indicate significant differences between Symbiodinium strains (P < 0.01, Tukey’s multiple range test)
Effect of glycosidase treatment and carbohydrates on Symbiodinium acquisition by juvenile polyps
To test the hypothesis of whether lectin, a carbohydrate binding protein, is involved in the recognition process between A. tenuis and Symbiodinium, we examined the effect of glycosidase treatment of Symbiodinium on its acquisition by juvenile polyps (Fig. 3a). The uptake of Symbiodinium by juvenile polyps was inhibited by glycopeptidase F-treatment (10.3 ± 11.6 cells/polyp) and by glycosidase mix treatment (20.0 ± 5.7 cells/polyp) (Fig. 3a). There were significant differences between the control (no treatment) and glycosidase treatments (P < 0.05, Dunnett’s multiple range test). These glycosidase treatments did not affect Symbiodinium motility.
Inhibition test of Symbiodinium acquisition activity by glycosidase treatment of Symbiodinium and the presence of carbohydrates. a Juvenile polyps were incubated with Symbiodinium strain NBRC102920 after treatment with the glycosidase mix, glycopeptidase F and no treatment. After 24 h, the number of Symbiodinium per polyp was counted. Values are mean ± SD (n = 5). Treatments that differ from the control (no treatment) by one-way ANOVA and Dunnett’s multiple comparison test at P < 0.01 are indicated by **. b Juvenile polyps were incubated with different carbohydrates for 1 h followed by 6 h incubation with Symbiodinium. After 6 h, the number of Symbiodinium cells per juvenile polyps was counted using in vivo chlorophyll a fluorescence. Values are mean ± SD (n = 5)
Next, the effect of carbohydrates on Symbiodinium acquisition by juvenile polyps was examined. One-way ANOVA revealed that Symbiodinium acquisition was significantly different between different carbohydrates; however, Tukey’s multiple comparison test did not show significant differences in the number of Symbiodinium acquired among different carbohydrates (P > 0.05). The number of Symbiodinium acquired by juvenile polyps (38.3 ± 21.0 cells/polyp) tended to decrease after exposure to Gal (19.3 ± 10.3 cells/polyp), GlcNAc (22.2 ± 9.0 cells/polyp) and GalNAc (16.9 ± 12.6 cells/polyp) (Fig. 3b). Thus, it is plausible that polyps have Gal-, GlcNAc-, and GalNAc-binding lectins that are involved in Symbiodinium acquisition. Based on the report of the isolation of GalNAc-binding lectin from other coral species [24], we attempted to purify GalNAc-binding lectin from A. tenuis.
Identification of GalNAc-binding lectin
In the purification of GalNAc-binding lectin from A. tenuis crude extract using GalNAc-Sepharose 6B resin, the eluted fraction contained proteins of sizes 14.6 and 29.0 kDa as shown by SDS-PAGE (Fig. 4). On de novo sequencing using MALDI-TOF/MS, three trypsin-digested amino acid fragments of the 14.6 kDa protein (m/z 1378.77, 1463.86, and 1913.16) had sequences of EFEN (I/L) VSGVK, YDQW (I/L) (I/L) ASPR, and HVNTV (I/L) AR, and two trypsin-digested fragments of the 29.0 kDa protein (m/z 1330.86 and 1866.19) had sequences of VXXXGWHVFK and N (I/L) (I/L) FGVTAGK, where X indicates undetermined amino acid residues. Comparison of these partial amino acid sequences with those in a public putative protein database of A. digitifera by FASTS analysis showed the sequences of the 14.6 kDa protein from A. tenuis to be similar to a region corresponding to adi_v1.13780 of A. digitifera putative protein (E-value 6.7 × 10−6). The N-terminal amino acid sequence of adi_v1.13780 (20–99 aa) showed similarity to tripartite motif-containing protein 2 (424–499 aa) of the Pacific oyster Crassostrea gigas, which contains a neuraminidase motif. The sequence of the 29.0 kDa protein from A. tenuis showed similarity to a corresponding region of adi_v1.08085 of A. digitifera putative protein (E-value 7.6 × 10−8) and to GalNAc- and GlcNAc-binding lectin Tachylectin-2 TL-2 (corresponding peptide region: 47.7 % identity, Fig. 1), one of the horseshoe crab lectins [44]. Therefore, the 29.0 kDa protein was termed AtTL-2.
Determination of the cDNA sequence of AtTL-2
AtTL-2 cDNA was cloned using the RACE method. The nucleotide sequence of AtTL-2 open reading frame was 801 bp, and the deduced amino acid sequence of AtTL-2 coincided with that obtained by de novo sequencing at regions of 25–34 and 150–159 aa (Fig. 1). The percent identities of AtTL-2 with the deduced amino acid sequence of Tachylectin-2 and related proteins from A. tenuis, A. digitifera, and A. millepora were 48.1, 92.1, 89.5, and 91.4 %, respectively (Fig. 1).
The structure of Tachylectin-2 is composed of a five-bladed β-propeller structure with five tandem repeats (49–68 % identity) [44, 45], and AtTL-2 also consists of five tandem repeats (43–66 % identity) (Fig. 1). The partial amino acid sequence obtained by de novo sequencing of a peak at m/z 1866.19 was located close to the starting position of mature Tachylectin-2 (Val20) [44] (Fig. 1). As the trypsin digests of AtTL-2 had a peak at m/z 1866.19, which corresponded to the molecular weight of TPTCENLLFGVTAGK (m/z 1866.13) in the deduced amino acid sequences, Thr20 of AtTL-2 is likely a starting position of the mature protein.
Specificity and neutralization activity of the anti-Tachylectin-2 antibody
To examine AtTL-2 function in the acquisition of Symbiodinium by juvenile polyps, it is instructive to use an antibody in experimental tests. We tested whether the anti-Tachylectin-2 antibody specifically recognizes AtTL-2. This antibody did not react with fractions separated by GalNAc-Sepharose chromatography that were shown to contain AtTL-2 by western analysis but did react with AtTL-2 on dot blotting. Among the fractions separated by GalNAc-Sepharose 6B chromatography, anti-Tachylectin-2 antibodies reacted with the crude extract of A. tenuis and the eluted fraction, which contained AtTL-2, but it did not react with the flow-through fraction, which did not contain AtTL-2 (Fig. 5). These findings suggest that anti-Tachylectin-2 antibodies recognize AtTL-2.
Some antibodies have the capability to inhibit the activity of proteins. Therefore, we investigated whether anti-Tachylectin-2 antibodies inhibit the binding activity of AtTL-2. When A. tenuis crude extract was separated on GalNAc-Sepharose 6B resin, the eluted fraction contained two proteins of sizes 14.6 and 29.0 kDa, similar to those shown in Fig. 4 (Fig. 6). The fraction also contained contaminant bands ranging in size from 50 to 66 kDa, which also appeared in sample buffer without proteins. On the other hand, when A. tenuis crude extract preincubated with anti-Tachylectin-2 antibodies was separated on GalNAc-Sepharose 6B resin, only the AtTL-2 of size 29.0 kDa disappeared from the eluted fraction (Fig. 6), indicating that anti-Tachylectin-2 antibodies inhibited the carbohydrate-binding activity of AtTL-2. Moreover, this result also shows that anti-Tachylectin-2 antibodies specifically recognized AtTL-2.
Inhibition of the carbohydrate-binding activity of AtTL-2 by anti-Tachylectin-2 antibodies. Crude extract of A. tenuis was mixed with or without anti-Tachylectin-2 antibodies and incubated at 4 °C for 1 h. These mixtures were subjected to batch GalNAc affinity chromatography. The eluted fractions for treatment without any antibody (−) or with anti-Tachylectin-2 antibodies (+) were analyzed by SDS-PAGE. M indicates protein standards. An arrow indicates the region containing AtTL-2
Participation of AtTL-2 in acquisition of Symbiodinium by juvenile polyps
We examined the effect of anti-Tachylectin-2 antibodies on the acquisition of Symbiodinium by juvenile polyps. The number of Symbiodinium acquired by polyps in the presence of anti-Tachylectin-2 antibodies (4.8 ± 3.8 cells/polyp, P < 0.05, Dunnett’s multiple range test) was significantly reduced to less than 23 % of that without any antibodies (20.5 ± 8.4 cells/polyp) (Fig. 7).
Inhibition of Symbiodinium acquisition activity by anti-Tachylectin-2 (TL-2) antibodies. Juvenile polyps were incubated without any antibodies, with control rabbit IgG, or with anti-TL-2 antibodies for 1 h. Then, polyps were incubated with Symbiodinium cells for 6 h. The number of Symbiodinium cells in juvenile polyp was counted using in vivo chlorophyll a fluorescence. Values are mean ± SD (n = 5). The treatment with antibodies that differs from the control (treatment with no antibodies) by one-way ANOVA with Dunnett’s multiple comparison test at P < 0.01 are indicated by **
Discussion
Acquisition of Symbiodinium strains NBRC102920 (clade A) and CCMP2556 (clade D) by juvenile A. tenuis polyps was highest, followed by the acquisition of strains CCMP2467, AJIS2-C2 and CS-161 (clade A) (Fig. 1b). This finding corroborates previous studies showing that Symbiodinium from different clades have physiologically different function [46–48]. A previous study by Yamashita et al. [34, 49] also showed that polyps of Acropora corals in natural environments mainly acquired Symbiodinium from clade A and/or D. Although a few clade C Symbiodinium were found in Acropora juvenile polyps in natural environments [49, 50], no strains from clade C were included in the present study due to the immobility of these strains in our laboratory culturing conditions. The Symbiodinium acquisition model developed here is expected to reflect the selectivity observed in natural environments. Symbiodinium acquisition decreased with an increase in rearing period (data not shown), and this may have led to the production of controls with different values for Symbiodinium acquisition (Figs. 2a, b, 3a, b).
Each Symbiodinium genetic clade was further divided into many types [51, 52]. Interestingly, differences in acquisition were observed even among closely related Symbiodinium in clade A: NBRC102920 (type A3), CCMP2467 (type A1), AJIIS2-C2 (type A1), CS-161 (type A3), and GTP-A6-Sy (type A2 relative) (Fig. 2b). Similarly, varying densities of strains from clade A were also found in larvae of A. tenuis [49]. The glycan profile of the Symbiodinium cell surface does not appear to depend on the clade assignment of Symbiodinium [53], suggesting that glycosylation patterns on the surface of Symbiodinium cells are more important than clade assignment in the acquisition of Symbiodinium by corals.
Lectin and glycosidase treatments targeting the cell surface of Symbiodinium inhibited the acquisition of Symbiodinium by the hosts Aiptasia pulchella and Fungia scutaria [11, 12]. In the present study, the acquisition of Symbiodinium by A. tenuis polyps was also inhibited by glycosidase treatment of Symbiodinium (Fig. 3a), suggesting that particular carbohydrate chains on the Symbiodinium cell surface play an important role in the acquisition process.
Based on the observed decrease of Symbiodinium acquisition by GalNAc, a GalNAc-binding lectin was separated from A. tenuis crude extract using GalNAc-Sepharose 6B resin (Fig. 4). The eluate contained two proteins with molecular masses of 14.6 and 29.0 kDa. The 29.0 kDa protein (AtTL-2) showed high similarity with Tachylectin-2. Tachylectin-2, one of the lectins from the horseshoe crab T. tridentatus, binds to GalNAc and GlcNAc [44]. In addition, Tachylectin-2 agglutinates bacteria, and the hemagglutinating activity is inhibited by some lipopolysaccharides and lipoteichoic acids [43, 54]. It has been proposed that Tachylectin-2 is involved in the immunity of horseshoe crab, such as in opsonization or in the blocking of adhesion of pathogens to the host cells [45]. Since some lectins have functions that are related not only to immune system functioning, but also to symbiosis [55], it is possible that AtTL-2 plays a role in the symbiosis between A. tenuis and Symbiodinium. Recently, the distribution of several coral lectins was determined [23, 26, 56], but their roles in symbiotic relationships remain to be clarified. Based on the inhibition of Symbiodinium acquisition by A. tenuis polyps by anti-Tachylectin-2 antibodies (Fig. 7), our results first clarified the important role of coral lectins in symbiosis; that is, AtTL-2 is involved in the acquisition step of symbiosis establishment between corals and Symbiodinium.
Tachylectin-2 homologues have been found in the corals Montastrea feveolata, Oculina diffusa, O. robusta, O. varicosa, Acropora palmata, A. digitifera, and A. millepora [57–60], but the function of Tachylectin-2 homologues in corals remains unknown. Our study revealed that AtTL-2, a Tachylectin-2 homologue, plays a role in the acquisition of Symbiodinium. AtTL-2 may bind to Symbiodinium and promote the acquisition of Symbiodinium by A. tenuis. Future studies will investigate the localization of AtTL-2 and the effect of AtTL-2 on Symbiodinium in order to clarify the role of AtTL-2 in symbiosis.
References
Stat M, Carter D, Hoegh-Guldberg O (2006) The evolutionary history of Symbiodinium and scleractinian hosts—Symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol Syst 8:23–43
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phycol 37:866–880
Pochon X, Montoya-Burgos JI, Stadelmann B, Pawlowski J (2006) Molecular phylogeny, evolutionary rates, and divergence timing of the symbiotic dinoflagellate genus Symbiodinium. Mol Phylogenet Evol 38:20–30
Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56:492–497
Santos SR, Taylor DJ, Kinzie RA, Hidaka M, Sakai K, Coffroth MA (2002) Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)-rDNA sequences. Mol Phylogenet Evol 23:97–111
Fitt WK, Chang SS, Trench RK (1981) Motility patterns of different strains of the symbiotic dinoflagellate Symbiodinium (= Gymnodinium) microadriaticum (Freudenthal) in culture. Bull Mar Sci 31:436–443
Davies P (1984) The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs 2:181–186
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571
Lin KL, Wang JT, Fang LS (2000) Participation of glycoproteins on zooxanthellal cell walls in the establishment of a symbiotic relationship with the sea anemone, Aiptasia pulchella. Zool Stud 39:172–178
Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM (2006) Lectin/glycan interactions play a role in recognition in a coral/dinoflagellate symbiosis. Cell Microbiol 8:1985–1993
Sharon N, Lis H (2004) History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14:53R–62R
Bulgheresi S, Schabussova I, Chen T, Mullin NP, Maizels RM, Ott JA (2006) A new C-type lectin similar to the human immunoreceptor DC-SIGN mediates symbiont acquisition by a marine nematode. Appl Environ Microbiol 72:2950–2956
Hirsch AM (1999) Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr Opin Plant Biol 2:320–326
Müller WE, Zahn RK, Kurelec B, Lucu C, Müller I, Uhlenbruck G (1981) Lectin, a possible basis for symbiosis between bacteria and sponges. J Bacteriol 145:548–558
Goto R, Kojii M, Yamazaki M, Kamiya H (1992) Purification and characterization of an agglutinin of the soft coral Sinularia species. Dev Comp Immunol 16:9–17
Kljajić Z, Schröder HC, Rottmann M, Cuperlović M, Movsesian M, Uhlenbruck G, Gasić M, Zahn RK, Müller WE (1987) A d-mannose-specific lectin from Gerardia savaglia that inhibits nucleocytoplasmic transport of mRNA. Eur J Biochem 169:97–104
Koch OM, Lee CK, Uhlenbruck G (1982) Cerianthin lectins: a new group of agglutinins from Cerianthus membranaceus (Singapore). Immunobiol 163:53–62
Müller W, Conrad J, Schröder HC, Zahn RK, Kljajić Z, Müller I, Uhlenbruck G (1983) Cell-cell recognition system in gorgonians: description of the basic mechanism. Mar Biol 76:1–6
Goto-Nance R, Muramoto K, Zenpo Y, Kamiya H (1996) Purification and characterization of the lectins of the soft coral Lobophytum variatum. Fish Sci 62:297–301
Jimbo M, Suda Y, Koike K, Nakamura-Tsuruta S, Kominami J, Kamei M, Hirabayashi J, Sakai R, Kamiya H (2013) Possible involvement of glycolipids in lectin-mediated cellular transformation of symbiotic microalgae in corals. J Exp Mar Biol Ecol 439:129–135
Jimbo M, Yanohara T, Koike K, Koike K, Sakai R, Muramoto K, Kamiya H (2000) The d-galactose-binding lectin of the octocoral Sinularia lochmodes: characterization and possible relationship to the symbiotic dinoflagellates. Comp Biochem Physiol B 125:227–236
Koike K, Jimbo M, Sakai R, Kaeriyama M, Kojii M, Ogata T, Maruyama T, Kamiya H (2004) Octocoral chemical signaling selects and controls dinoflagellate symbionts. Biol Bull 207:80–86
Jimbo M, Yamashita H, Koike K, Sakai R, Kamiya H (2010) Effects of lectin in the scleractinian coral Ctenactis echinata on symbiotic zooxanthellae. Fish Sci 76:355–363
Vidal-Dupiol J, Adjeroud M, Roger E, Foure L, Duval D, Mone Y, Ferrier-Pages C, Tambutte E, Tambutte S, Zoccola D, Allemand D, Mitta G (2009) Coral bleaching under thermal stress: putative involvement of host/symbiont recognition mechanisms. BMC Physiol 9:14
Kvennefors ECE, Leggat W, Hoegh-Guldberg O, Degnan BM, Barnes AC (2008) An ancient and variable mannose-binding lectin from the coral Acropora millepora binds both pathogens and symbionts. Dev Comp Immunol 32:1582–1592
Yuyama I, Hayakawa H, Endo H, Iwao K, Takeyama H, Maruyama T, Watanabe T (2005) Identification of symbiotically expressed coral mRNAs using a model infection system. Biochem Biophys Res Commun 336:793–798
Harii S, Nadaoka K, Yamamoto M, Iwao K (2007) Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar Ecol Prog Ser 346:89–96
Nishikawa A, Katoh M, Sakai K (2003) Larval settlement rates and gene flow of broadcast-spawning (Acropora tenuis) and planula-brooding (Stylophora pistillata) corals. Mar Ecol Prog Ser 256:87–97
Iwao K, Fujisawa T, Hatta M (2002) A cnidarian neuropeptide of the GLWamide family induces metamorphosis of reef-building corals in the genus Acropora. Coral Reefs 21:127–129
Hayashibara T, Iwao K, Omori M (2004) Induction and control of spawning in Okinawan staghorn corals. Coral Reefs 23:406–409
Yamashita H, Koike K (2013) Genetic identity of free-living Symbiodinium obtained over a broad latitudinal range in the Japanese coast. Phycol Res 61:68–80
Yamashita H, Suzuki G, Hayashibara T, Koike K (2013) Acropora recruits harbor “rare” Symbiodinium in the environmental pool. Coral Reefs 32:355–366
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Fernandez-Patron C, Castellanos-Serra L (1992) Reverse staining of sodium dodecyl sulfate polyacrylamide gels by imidazole-zinc salts: sensitive detection of unmodified proteins. Biotechniques 12:564–573
Beardsley RL, Reilly JP (2002) Optimization of guanidination procedures for MALDI mass mapping. Anal Chem 74:1884–1890
Chen P, Nie S, Mi W, Wang X-C, Liang S-P (2004) De novo sequencing of tryptic peptides sulfonated by 4-sulfophenyl isothiocyanate for unambiguous protein identification using post-source decay matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom 18:191–198
Strohalm M, Hassman M, Kosata B, Kodícek M (2008) mMass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun Mass Spectr 22:905–908
Mackey AJ, Haystead TAJ, Pearson WR (2002) Getting more from less: algorithms for rapid protein identification with multiple short peptide sequences. Mol Cell Proteomics 1:139–147
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Inamori K, Saito T, Iwaki D, Nagira T, Iwanaga S, Arisaka F, Kawabata S (1999) A newly identified horseshoe crab lectin with specificity for blood group A antigen recognizes specific O-antigens of bacterial lipopolysaccharides. J Biol Chem 274:3272–3278
Okino N, Kawabata S, Saito T, Hirata M, Takagi T, Iwanaga S (1995) Purification, characterization, and cDNA cloning of a 27-kDa lectin (L10) from horseshoe crab hemocytes. J Biol Chem 270:31008–31015
Beisel H-G, Kawabata S, Iwanaga S, Huber R, Bode W (1999) Tachylectin-2: crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J 18:2313–2322
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc R Soc 273:2305–2312
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138:1175–1181
Yamashita H, Suzuki G, Kai S, Hayashibara T, Koike K (2014) Establishment of coral-algal symbiosis requires attraction and selection. PLoS One 9:e97003
Abrego D, van Oppen MJH, Willis BL (2009) Onset of algal endosymbiont specificity varies among closely related species of Acropora corals during early ontogeny. Mol Ecol 18:3532–3543
Coffroth MA, Santos SR (2005) Genetic Diversity of Symbiotic Dinoflagellates in the Genus Symbiodinium. Protist 156:19–34
LaJeunesse TC (2005) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
Logan DDK, LaFlamme AC, Weis VM, Davy SK (2010) Flow-cytometric characterization of the cell-surface glycans of symbiotic dinoflagellates (Symbiodinium spp.). J Phycol 46:525–533
Kawabata S, Iwanaga S (1999) Role of lectins in the innate immunity of horseshoe crab. Dev Comp Immunol 23:391–400
Gross R, Vavre F, Heddi A, Hurst GD, Zchori-Fein E, Bourtzis K (2009) Immunity and symbiosis. Mol Microbiol 73:751–759
Kvennefors ECE, Leggat W, Kerr CC, Ainsworth TD, Hoegh-Guldberg O, Barnes AC (2010) Analysis of evolutionarily conserved innate immune components in coral links immunity and symbiosis. Dev Comp Immunol 34:1219–1229
Eytan RI, Hayes M, Arbour-Reily P, Miller M, Hellberg ME (2009) Nuclear sequences reveal mid-range isolation of an imperilled deep-water coral population. Mol Ecol 18:2375–2389
Meyer E, Aglyamova GV, Wang S, Buchanan-Carter J, Abrego D, Colbourne JK, Willis BL, Matz MV (2009) Sequencing and de novo analysis of a coral larval transcriptome using 454 GSFlx. BMC Genom 10:219
Schwarz JA, Brokstein PB, Voolstra C, Terry AY, Manohar CF, Miller DJ, Szmant AM, Coffroth MA, Medina M (2008) Coral life history and symbiosis: functional genomic resources for two reef building Caribbean corals, Acropora palmata and Montastraea faveolata. BMC Genom 9:97
Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T, Fujiyama A, Miller DJ, Satoh N (2011) Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476:320–323
Acknowledgments
We are grateful to Dr. Shun-ichiro Kawabata, Department of Sciences, the University of Kyushu, for providing anti-Tachylectin-2 antibodies. We thank Dr. Go Suzuki, Seikai National Fisheries Research Institute, Fisheries Research Agency for collecting A. tenuis. This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to MJ [10009069].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kuniya, N., Jimbo, M., Tanimoto, F. et al. Possible involvement of Tachylectin-2-like lectin from Acropora tenuis in the process of Symbiodinium acquisition. Fish Sci 81, 473–483 (2015). https://doi.org/10.1007/s12562-015-0862-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0862-y