Abstract
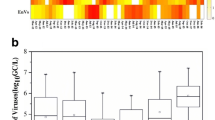
During the Amazonian flood season in 2012, the Negro River reached its highest level in 110 years, submerging residential and commercial areas which appeared associated with an elevation in the observed gastroenteritis cases in the city of Manaus. The aim of this study was to evaluate the microbiological water quality of the Negro River basin during this extreme flood to investigate this apparent association between the illness cases and the population exposed to the contaminated waters. Forty water samples were collected and analysed for classic and emerging enteric viruses. Human adenoviruses, group A rotaviruses and genogroup II noroviruses were detected in 100, 77.5 and 27.5% of the samples, respectively, in concentrations of 103–106 GC/L. All samples were compliant with local bacteriological standards. HAdV2 and 41 and RVA G2, P[6], and P[8] were characterised. Astroviruses, sapoviruses, genogroup IV noroviruses, klasseviruses, bocaviruses and aichiviruses were not detected. Statistical analyses showed correlations between river stage level and reported gastroenteritis cases and, also, significant differences between virus concentrations during this extreme event when compared with normal dry seasons and previous flood seasons of the Negro River. These findings suggest an association between the extreme flood experienced and gastrointestinal cases in the affected areas providing circumstantial evidence of causality between the elevations in enteric viruses in surface waters and reported illness.






Similar content being viewed by others
References
Ahern, M., Kovats, R. S., Wilkinson, P., Few, R., & Matthies, S. (2005). Global health impacts of floods: epidemiologic evidence. Epidemiologic Reviews, 27, 36–46.
Ahmed, M. U., Urasawa, S., Taniguchi, K., Urasawa, T., Kobayashi, N., Wakasugi, F., et al. (1991). Analysis of human rotavirus strains prevailing in Bangladesh in relation to nationwide floods brought by the 1988 monsoon. Journal of Clinical Microbiology, 29(10), 2273–2279.
Alderman, K., Turner, L. R., & Tong, S. (2012). Floods and human health: A systematic review. Environment International, 47, 37–47.
Allard, A., Albinsson, B., & Wadell, G. (2001). Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. Journal of Clinical Microbiology, 39(2), 498–505.
Amaral, M. S., Estevam, G. K., Penatti, M., Lafontaine, R., Lima, I. C., Spada, P. K., et al. (2015). The prevalence of norovirus, astrovirus and adenovirus infections among hospitalised children with acute gastroenteritis in Porto Velho, state of Rondônia, western Brazilian Amazon. Memórias do Instituto Oswaldo Cruz, 110(2), 215–221.
Beuret, C., Kohler, D., Baumgartner, A., & Lüthi, T. M. (2002). Norwalk-like virus sequences in mineral waters: One-year monitoring of three brands. Applied and Environmental Microbiology, 68(4), 1925–1931.
Boehm, A. B., Grant, S. B., Kim, J. H., Mowbray, S. L., McGee, C. D., Clark, C. D., et al. (2002). Decadal and shorter period variability of surf zone water quality at Huntington Beach, California. Environmental Science and Technology, 36(18), 3885–3892.
Bofill-Mas, S., Rusiñol, M., Fernandez-Cassi, X., Carratalà, A., Hundesa, A., & Girones, R. (2013). Quantification of human and animal viruses to differentiate the origin of the fecal contamination present in environmental samples. Biomed Research International, 2013, 192089.
Calgua, B., Fumian, T., Rusiñol, M., Rodriguez-Manzano, J., Mbayed, V. A., Bofill-Mas, S., et al. (2013). Detection and quantification of classic and emerging viruses by skimmed-milk flocculation and PCR in river water from two geographical areas. Water Research, 47(8), 2797–2810.
Campos, G. S., Silva Sampaio, M. L., Menezes, A. D., Tigre, D. M., Moura Costa, L. F., Chinalia, F. A., et al. (2016). Human bocavirus in acute gastroenteritis in children in Brazil. Journal of Medical Virology, 88(1), 166–170.
Cann, K. F., Thomas, D. R., Salmon, R. L., Wyn-Jones, A. P., & Kay, D. (2013). Extreme water-related weather events and waterborne disease. Epidemiology and Infection, 141(4), 671–686.
Carvalho-Costa, F. A., Araújo, I. T., Santos de Assis, R. M., Fialho, A. M., de Assis Martins, C. M., Bóia, M. N., et al. (2009). Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerging Infectious Diseases, 15(1), 95–97.
Carvalho-Costa, F. A., Mello Volotão, E., de Assis, R. M., Fialho, A. M., de Andrade, J. D. S., Rocha, L. N., et al. (2011). Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005-2009. The Pediatric Infectious Disease Journal, 30(1 Suppl), S35–S41.
CONAMA—National Environment Council (Conselho Nacional do Meio Ambiente). (2000). Ministério do Meio Ambiente. Resolução 274 de 29 de novembro de 2000. Diário Oficial da República Federativa do Brasil, Poder Executivo, Brasília, DF.
Couceiro, S. R. M., Hamada, N., Luz, S. L. B., Forsberg, B. R., & Pimentel, T. P. (2007). Deforestation and sewage effects on aquatic macroinvertebrates in urban streams in Manaus, Amazonas, Brazil. Hydrobiologia, 575(Issue 1), 271–284.
da Silva Assis, M. R., Vieira, C. B., Fioretti, J. M., Rocha, M. S., de Almeida, P. I., Miagostovich, M. P., et al. (2016). Detection and molecular characterization of gemycircularvirus from environmental samples in Brazil. Food and Environmental Virology, 8, 305–309. In press.
Dai, Y. C., Xu, Q. H., Wu, X. B., Hu, G. F., Tang, Y. L., Li, J. D., et al. (2010). Development of real-time and nested RT-PCR to detect astrovirus and one-year survey of astrovirus in Jiangmen City, China. Archives of Virology, 155(6), 977–982.
Das, B. K., Gentsch, J. R., Cicirello, H. G., Woods, P. A., Gupta, A., Ramachandran, M., et al. (1994). Characterization of rotavirus strains from newborns in New Delhi, India. Journal of Clinical Microbiology, 32(7), 1820–1822.
de Man, H., van den Berg, H. H., Leenen, E. J., Schijven, J. F., Schets, F. M., van der Vliet, J. C., et al. (2014). Quantitative assessment of infection risk from exposure to waterborne pathogens in urban floodwater. Water Research, 48, 90–99.
Ding, G., Zhang, Y., Gao, L., Ma, W., Li, X., Liu, J., et al. (2013). Quantitative analysis of burden of infectious diarrhea associated with floods in northwest of Anhui province, china: a mixed method evaluation. PLoS ONE, 8(6), e65112.
Ferreira, F. F., Guimarães, F. R., Fumian, T. M., Victoria, M., Vieira, C. B., Luz, S., et al. (2009). Environmental dissemination of group A rotavirus: P-type, G-type and subgroup characterization. Water Science and Technology, 60(3), 633–642.
Fewtrell, L., Kay, D., Watkins, J., Davies, C., & Francis, C. (2011). The microbiology of urban UK floodwaters and a quantitative microbial risk assessment of flooding and gastrointestinal illness. Journal of Flood Risk Management, 4, 77–87.
Fioretti, J. M., Rocha, M. S., Fumian, T. M., Ginuino, A., da Silva, T. P., de Assis, et al. (2016). Occurrence of human sapoviruses in wastewater and stool samples in Rio De Janeiro, Brazil. Journal of Applied Microbiology, 121(3), 855–862.
Fischer, T. K., Steinsland, H., Molbak, K., Ca, R., Gentsch, J. R., Valentiner-Branth, P., et al. (2000). Genotype profiles of Rotavirus Strains from children in suburban community in Guinea-Bissau, Western Africa. Journal of Clinical Microbiology, 38(1), 264–267.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Frappart, F., Seyler, F., Martinez, J.-M., Leon, J. G., & Cazenave, A. (2005). Floodplain water storage in the Negro River basin estimated from microwave remote sensing of inundation area and water levels. Remote Sensing of Environment, 99, 387–399.
Fumian, T. M., Leite, J. P., Rose, T. L., Prado, T., & Miagostovich, M. P. (2011). One year environmental surveillance of rotavirus specie A (RVA) genotypes in circulation after the introduction of the Rotarix® vaccine in Rio de Janeiro, Brazil. Water Research, 45(17), 5755–5763.
Fumian, T. M., Vieira, C. B., Leite, J. P., & Miagostovich, M. P. (2013). Assessment of burden of virus agents in an urban sewage treatment plant in Rio de Janeiro, Brazil. Journal of Water and Health, 11(1), 110–119.
Fun, B. N., Unicomb, L., Rahim, Z., Banu, N. N., Podder, G., Clemens, J., et al. (1991). Rotavirus-associated diarrhea in rural Bangladesh: two-year study of incidence and serotype distribution. Journal of Clinical Microbiology, 29(7), 1359–1363.
Gentsch, J. R., Glass, R. I., Woods, P., Gouvea, V., Gorziglia, M., Flores, J., et al. (1992). Identification of group A rotavirus gene 4 types by polymerase chain reaction. Journal of Clinical Microbiology, 30(6), 1365–1373.
Gómez, M. M., da Silva, M. F., Zeller, M., Heylen, E., Matthijnssens, J., Ichihara, M. Y., et al. (2013). Phylogenetic analysis of G1P[6] group A rotavirus strains detected in Northeast Brazilian children fully vaccinated with Rotarix™. Infection, Genetics and Evolution, 19, 395–402.
Gouvea, V., Santos, N., & Timenetsky, M. C. (1994). Identification of bovine and porcine rotavirus G types by PCR. Journal of Clinical Microbiology, 32(5), 1338–1340.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Haramoto, E., Kitajima, M., & Otagiri, M. (2013). Development of a reverse transcription-quantitative PCR assay for detection of salivirus/klassevirus. Applied and Environmental Microbiology, 79(11), 3529–3532.
Hashizume, M., Wagatsuma, Y., Faruque, A. S., Hayashi, T., Hunter, P. R., Armstrong, B., et al. (2008). Factors determining vulnerability to diarrhea during and after severe floods in Bangladesh. Journal of Water and Health, 6(3), 323–332.
Hernandez, J. D., da Silva, L. D., Sousa Junior, E. C., de Lucena, M. S., da Soares, L., Mascarenhas, J. D., et al. (2016). Analysis of uncommon norovirus recombinants from Manaus, Amazon region, Brazil: GII.P22/GII.5, GII.P7/GII.6 and GII.Pg/GII.1. Infection, Genetics and Evolution, 39, 365–371.
Hernroth, B. E., Conden-Hansson, A. C., Rehnstam-Holm, A. S., Girones, R., & Allard, A. K. (2002). Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Applied and Environmental Microbiology, 68(9), 4523–4533.
Hewitt, J., Greening, G. E., Leonard, M., & Lewis, G. D. (2013). Evaluation of human adenovirus and human polyomavirus as indicators of human sewage contamination in the aquatic environment. Water Research, 47(17), 6750–6761.
IBGE—The Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística). (2015). http://www.cidades.ibge.gov.br/xtras/perfil.php?codmun=130260. Accessed 10 May 2015
John, D. E., & Rose, J. B. (2005). Review of factors affecting microbial survival in groundwater. Environmental Science and Technology, 39(19), 7345–7356.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Kantola, K., Sadeghi, M., Antikainen, J., Kirveskari, J., Delwart, E., Hedman, K., et al. (2010). Real-time quantitative PCR detection of four human bocaviruses. Journal of Clinical Microbiology, 48(11), 4044–4050. Erratum in (2011). Journal of Clinical Microbiology, 48(11), 4044–4050. Erratum in (2011) Journal of Clinical Microbiology 49, 4029.
Kitajima, M., Hata, A., Yamashita, T., Haramoto, E., Minagawa, H., & Katayama, H. (2013). Development of a reverse transcription-quantitative PCR system for detection and genotyping of aichi viruses in clinical and environmental samples. Applied and Environmental Microbiology, 79(13), 3952–3958.
Kojima, S., Kageyama, T., Fukushi, S., Hoshino, F. B., Shinohara, M., Uchida, K., et al. (2002). Genogroup-specific PCR primers for detection of Norwalk-like viruses. Journal of Virological Methods, 100(1–2), 107–114.
Kunii, O., Nakamura, S., Abdur, R., & Wakai, S. (2002). The impact on health and risk factors of the diarrhoea epidemics in the 1998 Bangladesh floods. Public Health, 116(2), 68–74.
Kuo, H. W., Chen, L. Z., & Shih, M. H. (2015). High prevalence of type 41 and high sequence diversity of partial hexon gene of human adenoviruses in municipal raw sewage and activated sludge. Journal of Applied Microbiology, 119(4), 1181–1195.
LaBelle, R. L., & Gerba, C. P. (1979). Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Applied and Environmental Microbiology, 38(1), 93–101.
Lee, C. S., Lee, C., Marion, J., Wang, Q., Saif, L., & Lee, J. (2014). Occurrence of human enteric viruses at freshwater beaches during swimming season and its link to water inflow. The Science of the Total Environment, 472, 757–766.
Leite, J. P., Carvalho-Costa, F. A., & Linhares, A. C. (2008). Group A rotavirus genotypes and the ongoing Brazilian experience: A review. Memórias do Instituto Oswaldo Cruz, 103(8), 745–753.
Linhares, A. C., & Justino, M. C. A. (2014). Rotavirus vaccination in Brazil: effectiveness and health impact seven years post-introduction. Expert Review of Vaccines, 13(1), 43–57.
Loisy, F., Atmar, R. L., Guillon, P., Le Cann, P., Pommepuy, M., & Le Guyader, F. S. (2005). Real-time RT-PCR for norovirus screening in shellfish. Journal of Virological Methods, 123(1), 1–7.
Lowe, D., Ebi, K. L., & Forsberg, B. (2013). Factors increasing vulnerability to health effects before, during and after floods. International Journal of Environmental Research and Public Health, 10(12), 7015–7067.
Maestri, R. P., Kaiano, J. H., Neri, D. L., Soares, L. D. S., Guerra, S. D. F., Oliveira, D. D. S., et al. (2012). Phylogenetic analysis of probable non-human genes of group A rotaviruses isolated from children with acute gastroenteritis in Belém, Brazil. Journal of Medical Virology, 84(12), 1993–2002.
Marengo, J. A., Borma, L. S., Rodriguez, D. A., Pinho, P., Soares, W. R., & Alves, L. M. (2013). Recent extremes of drought and flooding in Amazonia: Vulnerabilities and human adaptation. American Journal of Climate Change, 2, 87–96.
MDDA—Brazilian Program of Monitoring Acute Diarrheal Diseases (Programa Brasileiro de Monitorização das Doenças Diarreicas Agudas). (2015). Ministério da Saúde, Brasília, DF
Melo, T. (2014). http://g1.globo.com/am/amazonas/noticia/2013/05/manaus-tera-plano-de-enfrentamento-de-doencas-causadas-pela-cheia.html Accessed 10 May 2014.
Melo, G. Z. S., da Costa, C. A., & dos Santos, I. G. C. (2013). Diversidade molecular de rotavirus do grupo A na cidade de Manaus, estado do Amazonas, Brasil, 2004-2006. Epidemiologia e Serviços de Saúde, 22(2), 265–272.
Miagostovich, M. P., Ferreira, F. F. M., Guimarães, F. R., Fumian, T. M., Diniz-Mendes, L., Luz, S. L. B., et al. (2008). Molecular detection and characterization of gastroenteritis viruses occurring naturally in the stream waters of Manaus, Central Amazonia, Brazil. Applied and Environmental Microbiology, 74(2), 375–382.
Ngaosuwankul, N., Thippornchai, N., Yamashita, A., Vargas, R. E., Tunyong, W., Mahakunkijchareon, Y., et al. (2013). Detection and characterization of enteric viruses in flood water from the 2011 thai flood. Japanese Journal of Infectious Diseases, 66(5), 398–403.
Ogorzaly, L., Walczak, C., Galloux, M., Etienne, S., Gassilloud, B., & Cauchie, H. M. (2016). Human adenovirus diversity in water samples using a next-generation amplicon sequencing approach. Food and Environmental Virology, 7, 112–121. In press.
Oka, T., Katayma, K., Hansman, G. S., Kageyama, T., Ogawa, S., Wu, F. T., et al. (2006). Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. Journal of Medical Virology, 78(10), 1347–1353.
Oliveira, A., Mascarenhas, J. D. P., Soares, L. S., Guerra, S. F. S., Gabbay, Y. B., Sánches, N., et al. (2012). Rotavirus serotype distribution in northern Brazil trends over a 27 year period pre and post national vaccine introduction. Trials in Vaccinology, 1, 4–9.
Phanuwan, C., Takizawa, S., Oguma, K., Katayama, H., Yunika, A., & Ohgaki, S. (2006). Monitoring of human enteric viruses and coliform bacteria in waters after urban flood in Jakarta, Indonesia. Water Science and Technology, 54(3), 203–210.
Pina, S., Puig, M., Lucena, F., Jofre, J., & Girones, R. (1998). Viral pollution in the environment and in shellfish: Human adenovirus detection by PCR as an index of human viruses. Applied and Environmental Microbiology, 64(9), 3376–3382.
Port of Manaus (Porto de Manaus). (2015). http://www.portodemanaus.com.br/?pagina=niveis-maximo-minimo-do-rio-negro Accessed 10 May 2015.
PROSAMIM—Social and Environmental Program for the Igarapés in Manaus (Programa Ambiental e Social dos Igarapés de Manaus). (2004). Relatório de Impacto Ambiental—RIMA. Manaus, Igarapé do Educandos. Governo do Estado do Amazonas, Secretaria de Estado de Infra-estrutura e Concremat Engenharia: Amazonas, Brasil.
PROSAMIM—Social and Environmental Program for the Igarapés in Manaus (Programa Ambiental e Social dos Igarapés de Manaus). (2012). PROSAMIM III—Igarapé São Raimundo Projeto Executivo. Igarapé São Raimundo, RIMA—Relatório de Impacto Ambiental—REV. 01; Governo do Estado do Amazonas, Secretaria de Estado de Infra-estrutura, Unidade de Gerenciamento de Programa Social e Ambiental dos Igarapés de Manaus (UGPI) e Concremat Engenharia: Amazonas, Brasil.
Rohayem, J., Dumke, R., Jaeger, K., Schröter-Bobsin, U., Mogel, M., Kruse, A., et al. (2006). Assessing the risk of transmission of viral diseases in flooded areas: Viral load of the River Elbe in Dresden during the flood of August 2002. Intervirology, 49(6), 370–376.
Rusiñol, M., Fernandez-Cassi, X., Hundesa, A., Vieira, C., Kern, A., Eriksson, I., et al. (2014). Application of human and animal viral microbial source tracking tools in fresh and marine waters from five different geographical areas. Water Research, 59, 119–129.
Rusiñol, M., Fernandez-Cassi, X., Timoneda, N., Carratalà, A., Abril, J. F., Silvera, C., et al. (2015). Evidence of viral dissemination and seasonality in a Mediterranean river catchment: Implications for water pollution management. Journal of Environmental Management, 159, 58–67.
Santos, N., Mendes, G. S., Silva, R. C., Pena, G. A., Rojas, M., & Amorim, A. R. (2015). Salivirus and aichivirus A infections in children with gastroenteritis in Brazil. Clinical Microbiology & Infection, 21(8), 799.e1-3.
Satyamurty, P., Costa, C. P. W., Manzi, A. O., & Candido, L. A. (2013). A quick look at the 2012 record flood in the Amazon Basin. Geophysical Research Letters, 4(7), 1396–1401.
Schmid, D., Lederer, I., Much, P., Pichler, A. M., & Allerberger, F. (2005). Outbreak of norovirus infection associated with contaminated flood water, Salzburg, 2005. Euro Surveillance, 10(6), E050616.3.
Schnitzler, J., Benzler, J., Altmann, D., Mücke, I., & Krause, G. (2007). Survey on the population´s needs and the public health response during floods in Germany 2002. Journal of Public Health and Management Practice, 13(5), 461–464.
Schwartz, B. S., Harris, J. B., Khan, A. I., Larocque, R. C., Sack, D. A., Malek, M. A., et al. (2006). Diarrheal epidemics in Dhaka, Bagladesh, during three consecutive floods: 1988, 1998, and 2004. American Journal of Tropical Medicine and Hygiene, 74(6), 1067–1073.
Silva, L. D., Rodrigues, E. L., Lucena, M. S., Lima, I. C., Oliveira, D. D. S., Soares, L. S., et al. (2013). Detection of the pandemic norovirus variant GII.4 Sydney 2012 in Rio Branco, state of Acre, northern Brazil. Memórias do Instituto Oswaldo Cruz, 108(8), 1068–1070.
Siqueira, J. A., Linhares, A. D. C., Gonçalves, M. D. S., Carvalho, T. C., Justino, M. C., Mascarenhas, J. D., et al. (2013). Group A rotavirus and norovirus display sharply distinct seasonal profiles in Belém, northern Brazil. Memórias do Instituto Oswaldo Cruz, 108(5), 661–664.
Taylor, J., Lai, K. M., Davies, M., Clifton, D., Ridley, I., & Biddulph, P. (2011). Flood management: Prediction of microbial contamination in large-scale floods in urban environments. Environment International, 37, 1019–1029.
Teixeira, D. M., Hernandez, J. M., Silva, L. D., Oliveira, D. S., Spada, P. K., Gurjão, T. C., et al. (2016). Occurrence of norovirus GIV in environmental water samples from Belém City, Amazon Region, Brazil. Food and Environmental Virology, 8(1), 101–104.
Trujillo, A. A., McCaustland, K. A., Zheng, D. P., Hadley, L. A., Vaughn, G., Adams, S. M., et al. (2006). Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. Journal of Clinical Microbiology, 44(4), 1405–1412.
Vieira, C. B., de Abreu Corrêa, A., Jesus, M. S., Luz, S. L. B., Wyn-Jones, P., Kay, D., et al. (2016). Viruses surveillance under different season scenarios of the Negro River Basin, Amazonia, Brazil. Food and Environmental Virology, 8(1), 57–69.
Villar, J. C. E., Ronchail, J., Guyot, J. L., Cochonneau, G., Filizola, N., Waldo, L., et al. (2009). Spatio-temporal rainfall variability in the Amazon basin countries (Brazil, Peru, Bolivia, Colombia, and Ecuador). International Journal of Climatology, 29(11), 1574–1594.
Wade, T. J., Sandhu, S. K., Levy, D., Lee, S., LeChevallier, M. W., Katz, L., et al. (2004). Did a severe flood in the Midwest cause an increase in the incidence of gastrointestinal symptoms? American Journal of Epidemiology, 159(4), 398–405.
WHO—World Health Organization. (2009). WHO/IVB/08.17—Manual of rotavirus detection and characterization methods (pp. 59–99). Geneva: World Health Organization, Department of Immunization, Vaccines and Biologicals.
Yard, E. E., Murphy, M. W., Schneeberger, C., Narayanan, J., Hoo, E., Freiman, A., et al. (2014). Microbial and chemical contamination during and after flooding in the Ohio River-Kentucky. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 49(11), 1236–1243.
Zeng, S. Q., Halkosalo, A., Salminen, M., Szakal, E. D., Puustinen, L., & Vesikari, T. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Journal of Virological Methods, 153(2), 238–240.
Acknowledgements
This work was funded by Viroclime project (www.viroclime.org) as part of the European Union 7th Framework Programme for Research, contract number 243923. We thank the PDTIS DNA Sequence Platform staff at FIOCRUZ-RJ for technical support in sequencing reactions and the FIOCRUZ-Manaus team for helping with samplings. This research work is within the scope of the activities of FIOCRUZ as a collaborating centre of PAHO/WHO of Public and Environmental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vieira, C.B., de Abreu Corrêa, A., de Jesus, M.S. et al. The Impact of the Extreme Amazonian Flood Season on the Incidence of Viral Gastroenteritis Cases. Food Environ Virol 9, 195–207 (2017). https://doi.org/10.1007/s12560-017-9280-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9280-x