Abstract
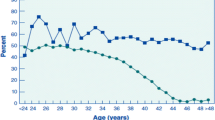
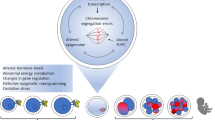
It is well established that age-related decline of a woman's fertility is related to the poor developmental potential of her gametes. The age-associated decline in female fertility is largely attributable to the oocyte aging caused by ovarian aging. Age-associated oocyte aging results in a decrease in oocyte quality. In contrast to ovarian aging, there is a concept of postovulatory oocyte aging. Postovulatory aging of oocytes, not being fertilized for a prolonged time after ovulation, is known to significantly affect the development of oocytes. Both categories of oocyte aging have similar phenotypes of reproductive failure. However, the mechanisms of the decline in oocyte quality are not necessarily equivalent. An age-dependent increase in aneuploidy is a key determinant of oocyte quality. The reduced expression of molecules regulating cell cycle control during meiosis might be involved in the age-dependent increase in aneuploidy. The mechanism of age-associated oocyte aging might be involved in mitochondrial dysfunction, whose etiologies are still unknown. Alternatively, the mechanism of postovulatory oocyte aging might be involved in reactive oxygen species-induced mitochondrial injury pathways followed by abnormal intracellular Ca2+ regulation of the endoplasmic reticulum. We suggest that future research into the mechanism of oocyte aging will be necessary to develop a method to rescue the poor developmental potential of aged oocytes.

Similar content being viewed by others
References
Balasch J. Ageing and infertility: an overview. Gynecol Endocrinol. 2010;26:855–60.
de Mouzon J, Lancaster P, Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson D. World collaborative report on assisted reproductive technology, 2002. Hum Reprod. 2009;24:2310–20.
Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, et al. Fertility and ageing. Hum Reprod Updat. 2005;11:261–76.
Leridon H. Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment. Hum Reprod. 2004;19:1548–53.
te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Updat. 2002;8:141–54.
Menken J, Trussell J, Larsen U. Age and infertility. Science. 1986;233:1389–94.
Alviggi C, Humaidan P, Howles CM, Tredway D, Hillier SG. Biological versus chronological ovarian age: implications for assisted reproductive technology. Reprod Biol Endocrinol. 2009;7:101.
Practice Committee of the American Society for Reproductive Medicine. Aging and infertility in women. Fertil Steril. 2006;86:S248–52.
Heffner LJ. Advanced maternal age–how old is too old? N Engl J Med. 2004;351:1927–9.
Sauer MV, Kavic SM. Oocyte and embryo donation 2006: reviewing two decades of innovation and controversy. Reprod Biomed Online. 2006;12:153–62.
Toner JP, Grainger DA, Frazier LM. Clinical outcomes among recipients of donated eggs: an analysis of the U.S. national experience, 1996–1998. Fertil Steril. 2002;78:1038–45.
Sakai N, Endo A. Effects of delayed mating on preimplantation embryos in spontaneously ovulated mice. Gamete Res. 1988;19:381–5.
Yanagimachi R, Chang MC. Fertilizable life of golden hamster ova and their morphological changes at the time of losing fertilizability. J Exp Zool. 1961;148:185–203.
Fissore RA, Kurokawa M, Knott J, Zhang M, Smyth J. Mechanisms underlying oocyte activation and postovulatory ageing. Reproduction. 2002;124:745–54.
Tarin JJ, Perez-Albala S, Cano A. Consequences on offspring of abnormal function in ageing gametes. Hum Reprod Updat. 2000;6:532–49.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Updat. 2009;15:573–85.
Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93.
Peluso JJ, Butcher RL. The effect of follicular aging on the ultrastructure of the rat oocyte. Fertil Steril. 1974;25:494–502.
Oussaid B, Lonergan P, Khatir H, Guler A, Monniaux D, Touze JL, Beckers JF, Cognie Y, Mermillod P. Effect of GnRH antagonist-induced prolonged follicular phase on follicular atresia and oocyte developmental competence in vitro in superovulated heifers. J Reprod Fertil. 2000;118:137–44.
Mihm M, Curran N, Hyttel P, Knight PG, Boland MP, Roche JF. Effect of dominant follicle persistence on follicular fluid oestradiol and inhibin and on oocyte maturation in heifers. J Reprod Fertil. 1999;116:293–304.
Downs SM. Induction of meiotic maturation in vivo in the mouse by IMP dehydrogenase inhibitors: effects on the developmental capacity of ova. Mol Reprod Dev. 1994;38:293–302.
Saito H, Koike K, Saito T, Nohara M, Kawagoe S, Hiroi M. Aging changes in the alignment of chromosomes after human chorionic gonadotropin stimulation may be a possible cause of decreased fertility in mice. Horm Res. 1993;39(Suppl 1):28–31.
Smith AL, Lodge JR. Interactions of aged gametes: in vitro fertilization using in vitro-aged sperm and in vivo-aged ova in the mouse. Gamete Res. 1987;16:47–56.
Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–7.
Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Updat. 2008;14:131–42.
Templeton A, Morris JK, Parslow W. Factors that affect outcome of in vitro fertilisation treatment. Lancet. 1996;348:1402–6.
Butcher RL, Collins WE, Fugo NW. Altered secretion of gonadotropins and steroids resulting from delayed ovulation in the rat. Endocrinology. 1975;96:576–86.
Hamaguchi H, Mikamo K. Morphological and cytogenetic studies on blastocysts following intrafollicular overripeness induced by delayed ovulation (author’s transl). Jinrui Idengaku Zasshi. 1974;19:88–9.
Huhtinen M, Koskinen E, Skidmore JA, Allen WR. Recovery rate and quality of embryos from mares inseminated after ovulation. Theriogenology. 1996;45:719–26.
Tarin JJ, Perez-Albala S, Aguilar A, Minarro J, Hermenegildo C, Cano A. Long-term effects of postovulatory aging of mouse oocytes on offspring: a two-generational study. Biol Reprod. 1999;61:1347–55.
Liang XW, Zhu JQ, Miao YL, Liu JH, Wei L, Lu SS, Hou Y, Schatten H, Lu KH, Sun QY. Loss of methylation imprint of Snrpn in postovulatory aging mouse oocyte. Biochem Biophys Res Commun. 2008;371:16–21.
Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342–6.
Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–48.
Morita Y, Maravei DV, Bergeron L, Wang S, Perez GI, Tsutsumi O, Taketani Y, Asano M, Horai R, Korsmeyer SJ, Iwakura Y, Yuan J, et al. Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ. 2001;8:614–20.
Matikainen T, Perez GI, Jurisicova A, Pru JK, Schlezinger JJ, Ryu HY, Laine J, Sakai T, Korsmeyer SJ, Casper RF, Sherr DH, Tilly JL. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet. 2001;28:355–60.
Pru JK, Tilly JL. Programmed cell death in the ovary: insights and future prospects using genetic technologies. Mol Endocrinol. 2001;15:845–53.
Matikainen T, Perez GI, Zheng TS, Kluzak TR, Rueda BR, Flavell RA, Tilly JL. Caspase-3 gene knockout defines cell lineage specificity for programmed cell death signaling in the ovary. Endocrinology. 2001;142:2468–80.
Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Updat. 2005;11:162–77.
Tarin JJ, Perez-Albala S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–50.
Fujino Y, Ozaki K, Yamamasu S, Ito F, Matsuoka I, Hayashi E, Nakamura H, Ogita S, Sato E, Inoue M. DNA fragmentation of oocytes in aged mice. Hum Reprod. 1996;11:1480–3.
Kuliev A, Cieslak J, Verlinsky Y. Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:193–8.
Pellestor F, Andreo B, Anahory T, Hamamah S. The occurrence of aneuploidy in human: lessons from the cytogenetic studies of human oocytes. Eur J Med Genet. 2006;49:103–16.
Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet Genome Res. 2005;111:206–12.
Steuerwald NM, Steuerwald MD, Mailhes JB. Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11:623–30.
Cukurcam S, Betzendahl I, Michel G, Vogt E, Hegele-Hartung C, Lindenthal B, Eichenlaub-Ritter U. Influence of follicular fluid meiosis-activating sterol on aneuploidy rate and precocious chromatid segregation in aged mouse oocytes. Hum Reprod. 2007;22:815–28.
Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat Genet. 2005;37:1351–5.
Gulyas BJ. Cortical granules of mammalian eggs. Int Rev Cytol. 1980;63:357–92.
Peluso JJ, England-Charlesworth C, Hutz R. Effect of age and of follicular aging on the preovulatory oocyte. Biol Reprod. 1980;22:999–1005.
Gallicano GI, Larabell CA, McGaughey RW, Capco DG. Novel cytoskeletal elements in mammalian eggs are composed of a unique arrangement of intermediate filaments. Mech Dev. 1994;45:211–26.
Szollosi D. Morphological changes in mouse eggs due to aging in the fallopian tube. Am J Anat. 1971;130:209–25.
Longo FJ. Ultrastructural changes in rabbit eggs aged in vivo. Biol Reprod. 1974;11:22–39.
Webb M, Howlett SK, Maro B. Parthenogenesis and cytoskeletal organization in ageing mouse eggs. J Embryol Exp Morphol. 1986;95:131–45.
Pickering SJ, Johnson MH, Braude PR, Houliston E. Cytoskeletal organization in fresh, aged and spontaneously activated human oocytes. Hum Reprod. 1988;3:978–89.
Kim NH, Moon SJ, Prather RS, Day BN. Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol Reprod Dev. 1996;43:513–8.
Longo FJ. Changes in the zones pellucidae and plasmalemma of aging mouse eggs. Biol Reprod. 1981;25:399–411.
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57:743–50.
Goud AP, Goud PT, Diamond MP, Van Oostveldt P, Hughes MR. Microtubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reprod Biomed Online. 2005;11:43–52.
Dodson MG, Minhas BS, Curtis SK, Palmer TV, Robertson JL. Spontaneous zona reaction in the mouse as a limiting factor for the time in which an oocyte may be fertilized. J In Vitro Fertil Embryo Transfer IVF. 1989;6:101–6.
Sundstrom P, Nilsson BO, Liedholm P, Larsson E. Ultrastructural characteristics of human oocytes fixed at follicular puncture or after culture. J In Vitro Fertil Embryo Transfer IVF. 1985;2:195–206.
Sun QY, Schatten H. Centrosome inheritance after fertilization and nuclear transfer in mammals. Adv Exp Med Biol. 2007;591:58–71.
Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction. 2006;131:193–205.
Sathananthan AH, Selvaraj K, Girijashankar ML, Ganesh V, Selvaraj P, Trounson AO. From oogonia to mature oocytes: inactivation of the maternal centrosome in humans. Microsc Res Tech. 2006;69:396–407.
Palacios MJ, Joshi HC, Simerly C, Schatten G. Gamma-tubulin reorganization during mouse fertilization and early development. J Cell Sci. 1993;104(Pt 2):383–9.
Eichenlaub-Ritter U, Stahl A, Luciani JM. The microtubular cytoskeleton and chromosomes of unfertilized human oocytes aged in vitro. Hum Genet. 1988;80:259–64.
Van Wissen B, Bomsel-Helmreich O, Debey P, Eisenberg C, Vautier D, Pennehouat G. Fertilization and ageing processes in non-divided human oocytes after GnRHa treatment: an analysis of individual oocytes. Hum Reprod. 1991;6:879–84.
Rodman TC. Chromatid disjunction in unfertilized ageing oocytes. Nature. 1971;233:191–3.
Mailhes JB, Young D, London SN. Postovulatory ageing of mouse oocytes in vivo and premature centromere separation and aneuploidy. Biol Reprod. 1998;58:1206–10.
Zenzes MT, Casper RF. Cytogenetics of human oocytes, zygotes, and embryos after in vitro fertilization. Hum Genet. 1992;88:367–75.
Schatten H. The mammalian centrosome and its functional significance. Histochem Cell Biol. 2008;129:667–86.
Cascio SM, Wassarman PM. Program of early development in the mammal: post-transcriptional control of a class of proteins synthesized by mouse oocytes and early embryos. Dev Biol. 1982;89:397–408.
Boerjan ML, de Boer P. First cell cycle of zygotes of the mouse derived from oocytes aged postovulation in vivo and fertilized in vivo. Mol Reprod Dev. 1990;25:155–63.
Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–52.
Takahashi T, Igarashi H, Kawagoe J, Amita M, Hara S, Kurachi H. Poor embryo development in mouse oocytes aged in vitro is associated with impaired calcium homeostasis. Biol Reprod. 2009;80:493–502.
Chi MM, Manchester JK, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biol Reprod. 1988;39:295–307.
Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, Kurachi H. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72:1256–61.
Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Tojo H, Toyoda Y. Inactivation of p34cdc2 kinase by the accumulation of its phosphorylated forms in porcine oocytes matured and aged in vitro. Zygote. 1999;7:173–9.
Tian XC, Lonergan P, Jeong BS, Evans AC, Yang X. Association of MPF. MAPK, and nuclear progression dynamics during activation of young and aged bovine oocytes. Mol Reprod Dev. 2002;62:132–8.
Tatone C, Carbone MC, Gallo R, Delle Monache S, Di Cola M, Alesse E, Amicarelli F. Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol Reprod. 2006;74:395–402.
Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, Wang WH. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005;72:373–83.
Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. J Cell Sci. 1992;103(Pt 2):389–96.
Igarashi H, Takahashi E, Hiroi M, Doi K. Aging-related changes in calcium oscillations in fertilized mouse oocytes. Mol Reprod Dev. 1997;48:383–90.
Takahashi T, Saito H, Hiroi M, Doi K, Takahashi E. Effects of aging on inositol 1,4,5-triphosphate-induced Ca(2+) release in unfertilized mouse oocytes. Mol Reprod Dev. 2000;55:299–306.
Jones KT, Whittingham DG. A comparison of sperm- and IP3-induced Ca2+ release in activated and aging mouse oocytes. Dev Biol. 1996;178:229–37.
Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30:438–64.
Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–4.
Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410.
Jagarlamudi K, Reddy P, Adhikari D, Liu K. Genetically modified mouse models for premature ovarian failure (POF). Mol Cell Endocrinol. 2010;315:1–10.
Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–24.
Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6.
Laissue P, Vinci G, Veitia RA, Fellous M. Recent advances in the study of genes involved in non-syndromic premature ovarian failure. Mol Cell Endocrinol. 2008;282:101–11.
Stolk L, Zhai G, van Meurs JB, Verbiest MM, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P, Soranzo N, de Keyzer JJ, et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet. 2009;41:645–7.
He C, Kraft P, Chen C, Buring JE, Pare G, Hankinson SE, Chanock SJ, Ridker PM, Hunter DJ, Chasman DI. Genome-wide association studies identify loci associated with age at menarche and age at natural menopause. Nat Genet. 2009;41:724–8.
Warburton D. Biological aging and the etiology of aneuploidy. Cytogenet Genome Res. 2005;111:266–72.
Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA. Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol Hum Reprod. 2001;7:49–55.
Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263–78.
Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–8.
Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod. 2010;25:957–68.
de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, te Velde ER. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod. 2004;70:419–24.
Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–17.
Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64:577–83.
Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod Biomed Online. 2000;1:96–100.
Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility—a clinician’s perspective. Reprod Biomed Online. 2005;11:641–50.
Tarin JJ, Gomez-Piquer V, Pertusa JF, Hermenegildo C, Cano A. Association of female aging with decreased parthenogenetic activation, raised MPF, and MAPKs activities and reduced levels of glutathione S-transferases activity and thiols in mouse oocytes. Mol Reprod Dev. 2004;69:402–10.
Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407.
Takase K, Ishikawa M, Hoshiai H. Apoptosis in the degeneration process of unfertilized mouse ova. Tohoku J Exp Med. 1995;175:69–76.
Exley GE, Tang C, McElhinny AS, Warner CM. Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Biol Reprod. 1999;61:231–9.
Spanos S, Rice S, Karagiannis P, Taylor D, Becker DL, Winston RM, Hardy K. Caspase activity and expression of cell death genes during development of human preimplantation embryos. Reproduction. 2002;124:353–63.
Rong Y, Distelhorst CW. Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol. 2008;70:73–91.
Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod. 2002;66:1828–37.
Miyazaki S, Ito M. Calcium signals for egg activation in mammals. J Pharmacol Sci. 2006;100:545–52.
Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128:917–28.
Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–91.
Toth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction. 2006;131:27–34.
Rogers NT, Halet G, Piao Y, Carroll J, Ko MS, Swann K. The absence of a Ca(2+) signal during mouse egg activation can affect parthenogenetic preimplantation development, gene expression patterns, and blastocyst quality. Reproduction. 2006;132:45–57.
Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–44.
Gordo AC, Wu H, He CL, Fissore RA. Injection of sperm cytosolic factor into mouse metaphase II oocytes induces different developmental fates according to the frequency of [Ca(2+)](i) oscillations and oocyte age. Biol Reprod. 2000;62:1370–9.
Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–30.
Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K. Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science. 1992;257:251–5.
Miyazaki S, Shirakawa H, Nakada K, Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993;158:62–78.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21.
East JM. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology (review). Mol Membr Biol. 2000;17:189–200.
Misquitta CM, Mack DP, Grover AK. Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium. 1999;25:277–90.
Dumollard R, Marangos P, Fitzharris G, Swann K, Duchen M, Carroll J. Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development. 2004;131:3057–67.
Dumollard R, Duchen M, Sardet C. Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol. 2006;17:314–23.
He H, Lam M, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell biol. 1997;138:1219–28.
Kuo TH, Kim HR, Zhu L, Yu Y, Lin HM, Tsang W. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene. 1998;17:1903–10.
Ramalho-Santos J, Varum S, Amaral S, Mota PC, Sousa AP, Amaral A. Mitochondrial functionality in reproduction: from gonads and gametes to embryos and embryonic stem cells. Hum Reprod Updat. 2009;15:553–72.
Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–8.
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis Int J Program Cell Death. 2007;12:913–22.
Rohn TT, Hinds TR, Vincenzi FF. Ion transport ATPases as targets for free radical damage. Protection by an aminosteroid of the Ca2+ pump ATPase and Na+/K+ pump ATPase of human red blood cell membranes. Biochem Pharmacol. 1993;46:525–34.
Wesson DE, Elliott SJ. The H2O2-generating enzyme, xanthine oxidase, decreases luminal Ca2+ content of the IP3-sensitive Ca2+ store in vascular endothelial cells. Microcirculation. 1995;2:195–203.
Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38:1571–5.
Li D, Ueta E, Kimura T, Yamamoto T, Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer science. 2004;95:644–50.
Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci USA. 2003;100:15035–40.
Acknowledgments
This study was supported by a Grant-in-aid for General Science Research No. 22591815 to Toshifumi Takahashi, 20591905 to Hideki Igarashi, 22390308 to Hirohisa Kurachi, and the Global COE Program for Medical Sciences from the Japan Society for the Promotion of Science.
Conflict of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Takahashi, T., Igarashi, H., Amita, M. et al. Cellular and molecular mechanisms of various types of oocyte aging. Reprod Med Biol 10, 239–249 (2011). https://doi.org/10.1007/s12522-011-0099-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-011-0099-0