Abstract
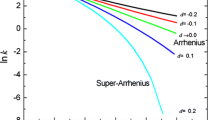
The most popular model of the temperature effect on the rate of chemical and biochemical reactions and biological processes in foods is the Arrhenius equation. Reactions and processes that have an optimal or threshold temperature, or whose rate constant versus the absolute temperature reciprocal plot is curvilinear, clearly do not follow the original Arrhenius model. Their kinetics has been described by expanded versions of the Arrhenius equation, the WLF model or log-logistic model and more recently by the Eyring–Polanyi equation. Computer simulations and re-analysis of published experimental rate–temperature relationships show that over a wide range of temperatures, the fit of the Arrhenius equation can be matched by a simpler exponential model, akin to the log-linear model used in food microbiology. This indicates that the temperature-independent energy of activation’s existence cannot be inferred from the Arrhenius plot’s linearity alone and needs independent verification. The same can be said about the Eyring–Polanyi model’s fit and the inference of a temperature-independent free energy of activation. These two models’ good fit might be primarily due to their mathematical structure and not the validity of their underlying statistical mechanistic assumptions. Similarly, the WLF model’s fit can be matched by other two-parameter empirical models that do not imply any analogy between the response of food systems to heat and the rheology of plasticized synthetic rubbery polymers. Therefore, parameters of temperature dependence models obtained by curve fitting should not be considered as having physical meaning unless verified experimentally by an independent test.











Similar content being viewed by others
References
Abiad MG, Carvajal MT, Campanella OH (2009) A review on methods and theories to describe the glass transition phenomenon: applications in food and pharmaceutical products. Food Eng Rev 1:105–132
Cisse M, Valliant F, Acosta O, Dhuique-Mayer C, Dornier M (2009) Thermal degradation kinetics of anthocyanins from blood orange, blackberry and reoselle using the Arrhenius, Eyring and Ball models. J Agric Food Chem 57:6285–6291
Donth EJ (2001) The glass transition: relaxation dynamics of liquids and disordered materials. Springer, New York
Fragoso CT, Battisti R, Miranda C, de Jesus PC (2009) Kinetic of the degradation of C.I. Food Yellow 3 and C.I. Food Yellow 4 azo dyes by the oxidation with hydrogen peroxide. J Mol Catal A: Chem 301:93–97
Giannakourou MC, Taoukis PS (2003) Stability of dehydrofrozen green peas pretreated with nonconventional osmotic agents. J Food Sci 68:2002–2010
Giovambattista N, Angell CA, Sciortino F, Stanley HE (2004) Glass-transition temperature of water: a simulation study. Phys Rev Lett 93:4
Huang L (2010) Growth kinetics of Escherichia coli O157:H7 in mechanically-tenderized beef. Int J Food Microbiol 140:40–48
Huang L (2011) Response to letter to the editor: growth kinetics of Escherichia coli O157:H7 in mechanically-tenderized beef. Int J Food Microbiol 147:81–82
Huang L, Hwang A, Phillips J (2011) Effect of temperature on microbial growth rate—mathematical analysis: the Arrhenius and Eyring–Ploany connection. J Food Sci 76:E533–E560
Langer J (2007) The mysterious glass transition. Phys Today (Feb. Issue): 8–9
McMeekin TA, Olley JN, Ross T, Ratkowsky DA (1993) Predictive microbiology: theory and application. Wiley, New York
McLeish T, Olmsted P, Hamley I (2001) Emerging themes in polymer theory. In: Ryan AJ (ed) Emerging themes in polymer science. Royal Society of Chemistry, Cambridge
Peleg M (1992) On the use of the WLF model in polymers and foods. Crit Rev Food Sci 32:59–66
Peleg M (1993) Glass transition and the physical stability of food powders. In: Blanshard JMV, Lilliford PJ (eds) The glassy state in foods. Nottingham University Press, Nottingham, pp 435–451
Peleg M (1996) On modeling changes in food and biosolids at and around their glass transition temperature range. CRC Crit Rev Food Sci Nutr 36:49–67
Peleg M (2006) Advanced quantitative microbiology for food and biosystems: models for predicting growth and inactivation. CRC Press, Boca Raton
Peleg M, Engel R, Gonzalez-Martinez C, Corradini MG (2002) Non- Arrhenius and non-WLF kinetics in food systems. J Sci Food Agric 82:1346–1355
Peleg M, Normand MD, Corradini MG (2012) The Arrhenius equation revisited. Crit Rev Food Sci Nutr 52:830–851
Ratkowsky DA, Lowry RK, McMeekin TA, Stokes AN, Chandler RE (1983) Model for bacterial growth rate throughout the entire biokinetic temperature range. J Bacteriol 154:1222–1226
Ratkowsky DA, Olley J, McMeekin TA, Ball A (1982) A relation between temperature and growth rate of bacterial cultures. J Bacteriol 149:1–5
Ratkowsky DA, Ross T, McMeekin TA, Olley J (1991) Comparison of Arrhenius-type and Belehradek-type models for the prediction of bacterial growth in foods. J Appl Bacteriol 71:452–459
Roos YH (1995) Phase transitions in foods. Academic Press, London
Roos YH (2010) Glass transition temperature and its relevance in food processing. Ann Rev Food Sci Technol 1:469–496
Ross EW (1993) Relation of bacterial destruction to chemical marker formation during processing by thermal pulses. J Food Process Eng 16:247–270
Ross T, Dalgaard P (2004) Secondary models. In: McKellar RC, Lu X (eds) Modeling microbial responses in food. CRC Press, Boca Raton, pp 64–150
Ross T, Olley J, McMeekin TA, Ratkowsky DA (2011) Some comments on Huang, L. (2010) Growth kinetics of Escherichia coli O157:H7 in mechanically tenderized beef. Int J Food Microbiol 147:78–80
Sapru V, Labuza TP (1993) Temperature-dependence of thermal inactivation rate constants of bacterial-spores in a glassy state. J Ind Microbiol 12:247–250
Seyler RJ (1994) Assignment of the glass transition. ASTM, Philadelphia
Slade L, Levine H (1991) Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit Rev Food Sci Nutr 30:115–360
Török T, King AD Jr (1991) Thermal inactivation of food-borne yeasts. J Food Sci 56:6–9
Van Boekel MAJS (2008) Kinetic modeling of food quality: a critical review. Compr Rev Food Sci Food Saf 7:144–158
Van Boekel MAJS (2009) Kinetic modeling of reactions in foods. CRC Press, Boca Raton
Velikov V, Borick S, Angell CA (2001) The glass transition of water, based on hyperquenching experiments. Science 294:2335–2338
Verbeyst L, Oey I, der Plancken IV, Hendrickx M, Loey AV (2010) Kinetic study on the thermal and pressure degradation of anthocyanins in strawberries. Food Chem 123:267–274
Acknowledgments
Contribution of the Massachusetts Agricultural Experiment Station, Amherst, MA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barsa, C.S., Normand, M.D. & Peleg, M. On Models of the Temperature Effect on the Rate of Chemical Reactions and Biological Processes in Foods. Food Eng Rev 4, 191–202 (2012). https://doi.org/10.1007/s12393-012-9056-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-012-9056-x