Abstract
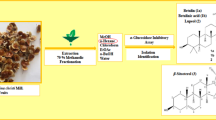
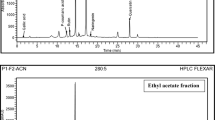
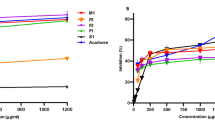
Sugarcane molasses is a wealthy source of health beneficial phenolic compounds and exhibited significant biological properties. Present study was designed to assess the inhibitory effect of sugarcane molasses on α-glucosidase and α-amylase. Hypoglycemic fractions were extracted using macro-porous and ion exchange resins. The total phenolics content was 0.179 ± 0.003 mg GAE per milligram of extract. Four phenolic acids which include caffeic acid (11.64 mg/g), ferulic acid (10.49 mg/g), chlorogenic acid (1.77 mg/g), and gallic acid (0.87 mg/g) were identified and quantified by high-performance liquid chromatography. The inhibitory activities of sugarcane molasses were 4.693 mg/mL (Km = 1.099 mL/mg) and 4.254 mg/mL (Km 0.238 mL/mg) for α-glucosidase and α-amylase, respectively, which revealed that sugarcane molasses could be a useful addition in medicinal preparations as nutraceutical and functional food for diabetic patients.




Similar content being viewed by others
References
Adisakwattana, S., P. Chantarasinlapin, H. Thammarat, and S. Yibchok-Anun. 2009. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. Journal of Enzyme Inhibition and Medicinal Chemistry 245: 194–200.
Adisakwattana, S., T. Ruengsamran, P. Kampa, and W. Sompong. 2012. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary and Alternative Medicine 12: 110–111.
Andjelkovic, M., J.V. Camp, B.D. Meulenaer, G. Depaemelaere, C. Socaciu, and M. Verloo. 2006. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chemistry 98: 23–31.
Burda, S., and W. Oleszek. 2001. Antioxidant and antiradical activities of flavonoids. Journal of Agriculture and Food Chemistry 49: 2774–2779.
Burlingham, B.T., and T.S. Widlanski. 2003. An intuitive look at the relationship of Ki and IC50: A more general use for the Dixon plot. Journal of Chemical Education 80: 214–218.
Chan, S., S. Kanchanatawee, and K. Jantama. 2012. Production of succinic acid from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Bioresource Technology 103: 329–336.
Department of Agriculture, Forestry and Fisheries, Okinawa Prefectural Government 2012. Annual report of sugarcane and sugar production in Okinawa prefecture in Japanese. 76–90.
De Sales, P.M., P.M. De Souza, L.A. Simeoni, P.O. Magalhães, and D. Silveira. 2012. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. Journal of Pharmacy and Pharmaceutical Sciences 15: 141–183.
Fan, P., L.A. Terrier, A. Hay, A. Marston, and K. Hostettmann. 2010. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F Shmidt ex Maxim Polygonaceae. Fitoterapia 81: 124–131.
Feng, S.M., Z.S. Luo, Y.B. Zhang, Z. Zhong, and B.Y. Lu. 2014. Phytochemical contents and antioxidant capacities of different parts of two sugarcane Saccharum officinarum L. cultivars. Food Chemistry 151: 452–458.
Ferreres, F., J. Vinholes, A. Gil-Izquierdo, P. Valentão, R.F. Gonçalves, and P.B. Andrade. 2013. In vitro studies of α-glucosidase inhibitors and antiradical constituents of Glandora diffusa (Lag.) DC Thomas infusion. Food Chemistry 136: 1390–1398.
Funke, I., and M.F. Melzig. 2005. Effect of different phenolic compounds on α-amylase activity: screening by microplate-reader based kinetic assay. Pharmazie 6010: 796–797.
Fu, X., S.J. Yu, Y.G. Min, and C.C. Chung. 2003. Extraction of natural antioxidants from sugarcane. Sugarcane and Canegar 5: 37–41.
Israili, Z.H. 2011. Advances in the treatment of type 2 diabetes mellitus. American Journal of Therapeutics 18: 117–152.
Jia, G.F., and Y.J. Di. 2012. Research advancement of separation and physiological activity of natural α-glucosidase inhibitor. Food and Nutrition in China 18: 65–68.
Kandra, L. 2003. α-amylase of medical and industrial importance. Journal of Molecular Structure: THEOCHEM 666: 487–498.
Kang, W.Y., L. Zhang, and Y.L. Song. 2009. α-glucosidase inhibitors from Luculiapinciana. China Journal of Chinese Material 344: 406–409.
Kim, J.S., T.K. Hyun, and M.J. Kim. 2011. The inhibitory effects of ethanol extracts from Sorghum. Food Chemistry 124: 1647–1651.
Lordan, S., T.J. Smyth, A. Soler-Vila, C. Stanton, and R.P. Ross. 2013. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chemistry 1413: 2170–2176.
Phan, M.A.T., J. Wang, J.Y. Tang, and Y.Z. Lee. 2013. Evaluation of α-glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. LWT—Food Science and Technology 53: 492–498.
Saito, N., H. Sakai, H. Sekihara, and Y. Yajima. 1998. Effect of an α-glucosidase inhibitor voglibose, in combination with sulphonilureas, on glycaemic control in type 2 diabetes patients. Journal of International Medical Research 26: 219–232.
Slinkard, K., and V.L. Singleton. 1977. Total phenol analyses: Automation and comparison with manual methods. American Journal of Enology and Viticulture 8: 49–55.
Stand, E., H.J. Baumgartl, M. Fuchtenbusch, and J. Stemplinger. 1999. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes, Obesity & Metabolism 1: 215–220.
Takara, K., K. Otsuka, K. Wada, H. Wasaki, and M. Yamashita. 2007. 1,1-Diphenyl-2-picrylhydrazyl radical scavenging activity and tyrosinase inhibitory effects of constituents of sugarcane molasses. Bioscience, Biotechnology, and Biochemistry 71: 183–191.
Tangphatsornruang, S., M. Naconsie, C. Thammarongtham, and J. Narangajavana. 2005. Isolation and characterization of an α-amylase gene in cassava Manihot esculenta. Plant Physiology and Biochemistry 43: 821–827.
van de Laar, F.A. 2008. α-Glucosidase inhibitors in the early treatment of type 2 diabetes. Vascular Health and Risk Management 4: 1189–1195.
Wang, B.S., L.W. Chang, Z.C. Kang, H.L. Chu, H.M. Tai, and M.H. Huang. 2011. Inhibitory effects of molasses on mutation and nitric oxide production. Food Chemistry 126: 1102–1107.
Wang, H., Y.J. Du, and M.C. Song. 2010. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chemistry 123: 6–13.
Whetten, R.W., J.J. MacKay, and R.R. Sederoff. 1998. Recent advances in understanding lignin biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 49: 585–609.
Zhang, A.J., A.M. Rimando, W. Fish, S.R. Mentreddy, and S.T. Mathews. 2012. Serviceberry [Amelanchier alnifolia (Nutt.) Nutt.ex. M. Roem (Rosaceae)] leaf extract inhibits mammalian α-glucosidase activity and suppresses postprandial glycemic response in a mouse model of diet-induced obesity and hyperglycemia. Journal of Ethnopharmacology 143: 481–487.
Acknowledgments
This work was supported by the ministry of science and technology in agriculture science and technology Achievements Transformation Fund Project (No. 2013GB23600669), the Science and Technology Planning Project of Guangdong Province, China (No. 2011B050400035), the Science and Technology Planning Project of Guang Zhou, China (No. 2013J4500036) and the Open Project Program of Provincial Key Laboratory of Green Processing Technology and Product Safety of Natural Products (201303).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kong, F., Yu, S., Zeng, F. et al. Phenolics Content and Inhibitory Effect of Sugarcane Molasses on α-Glucosidase and α-Amylase In Vitro. Sugar Tech 18, 333–339 (2016). https://doi.org/10.1007/s12355-015-0385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-015-0385-y