Abstract
Introduction
To evaluate the comparative efficacy and safety of ropinirole and pramipexole as adjunctive therapies to levodopa (l-dopa) for the management of advanced Parkinson’s disease (PD), via a systematic review and network meta-analysis.
Methods
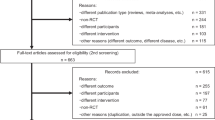
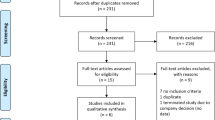
Twenty-one double-blind randomised controlled trials of patients with advanced PD with motor fluctuations receiving l-dopa comparing ropinirole or pramipexole with comparators were identified from 2550 publications. Bayesian indirect comparison methods were applied to independently review efficacy outcomes including off-time reduction, Unified Parkinson's Disease Rating Scale-Activity of Daily Living (UPDRS-ADL) and UPDRS-motor scores, and safety outcomes including adverse events (AE) and patient withdrawals, to determine indirect treatment comparison mean differences (MD) or hazard ratios (HR) with 95% confidence intervals (CI).
Results
The indirect efficacy comparison resulted in a statistically nonsignificant off-time reduction difference (hours) of ropinirole-sustained release (SR) versus pramipexole-immediate release (MD − 0.25; 95% CI − 0.71, 0.21) and ropinirole-SR versus pramipexole-extended release (ER) (MD 0.18; 95% CI − 0.40, 0.76). Ropinirole-SR adjunctive treatment showed a tendency towards more improvement in UPDRS-ADL score (MD 1.24; 95% CI 0.23, 2.24) than adjunctive treatment of pramipexole-ER. Pramipexole-ER may be less likely to induce somnolence as an AE compared with ropinirole-SR (HR 0.46; 95% CI 0.23, 0.89). However, there were no statistically significant differences in UPDRS-motor score reduction, incidence of dyskinesia, hallucination, hypotension, insomnia and nausea, or withdrawals due to AE, for any reason.
Conclusion
Adjunctive therapy with ropinirole-SR or pramipexole appears to offer similar efficacy and tolerability in patients with advanced PD on the basis of this indirect comparison.
Funding
GSK


Similar content being viewed by others
References
Poewe W. Parkinson disease. Nature Reviews Disease Primers. 2017;3:Article number: 17013
Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351(24):2498–508.
Stowe R, Ives N, Clarke CE, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010(7):CD007166.
Weber J, Keating GM. Ropinirole prolonged release: in advanced Parkinson's disease. CNS Drugs. 2009;23(1):81–90.
Pahwa R, Stacy MA, Factor SA, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007;68(14):1108–15.
Pinter MM, Pogarell O, Oertel WH. Efficacy, safety, and tolerance of the non-ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson's disease: a double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry. 1999;66(4):436–41.
Dias S, Welton NJ, Sutton AJ, Ades AE. A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: NICE Decision Support Unit Technical Support Documents; 2014.
Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–40.
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Inconsistency in networks of evidence based on randomised controlled trials. London: NICE Decision Support Unit Technical Support Documents; 2014.
Poewe WH, Rascol O, Quinn N, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6(6):513–20.
Wermuth L. A double-blind, placebo-controlled, randomized, multi-center study of pramipexole in advanced Parkinson's disease. Eur J Neurol. 1998;5(3):235–42.
Moller JC, Oertel WH, Koster J, Pezzoli G, Provinciali L. Long-term efficacy and safety of pramipexole in advanced Parkinson's disease: results from a European multicenter trial. Mov Disord. 2005;20(5):602–10.
Schapira AH, Barone P, Hauser RA, et al. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology. 2011;77(8):767–74.
Wong KS, Lu CS, Shan DE, Yang CC, Tsoi TH, Mok V. Efficacy, safety, and tolerability of pramipexole in untreated and levodopa-treated patients with Parkinson's disease. J Neurol Sci. 2003;216(1):81–7.
Lieberman A, Ranhosky A, Korts D. Clinical evaluation of pramipexole in advanced Parkinson's disease: results of a double-blind, placebo-controlled, parallel-group study. Neurology. 1997;49(1):162–8.
Guttman M. Double-blind comparison of pramipexole and bromocriptine treatment with placebo in advanced Parkinson's disease. International Pramipexole-Bromocriptine Study Group. Neurology. 1997;49(4):1060–5.
Rascol O, Lees AJ, Senard JM, Pirtosek Z, Montastruc JL, Fuell D. Ropinirole in the treatment of levodopa-induced motor fluctuations in patients with Parkinson's disease. Clin Neuropharmacol. 1996;19(3):234–45.
Lieberman A, Olanow CW, Sethi K, et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group. Neurology. 1998;51(4):1057–62.
Korczyn. Anti-Parkinson efficacy (L-dopa sparing effect) of ropinirole versus placebo as adjunct therapy in parkinsonian patients not optimally controlled on L-dopa. Confidential Report. 1990.
Stocchi F, Giorgi L, Hunter B, Schapira AH. PREPARED: Comparison of prolonged and immediate release ropinirole in advanced Parkinson's disease. Mov Disord. 2011;26(7):1259–65.
Mizuno Y, Yanagisawa N, Kuno S, et al. Randomized, double-blind study of pramipexole with placebo and bromocriptine in advanced Parkinson's disease. Mov Disord. 2003;18(10):1149–56.
Hattori N, Nishioka H, Hasegawa K. Comparison of ropinirole controlled- and immediate-release in Japanese patients with advanced Parkinson's disease. Neurol Clin Neurosci. 2015;3(1):18–24. https://doi.org/10.1111/ncn3.128.
Zesiewicz TA, Chriscoe S, Jimenez T, Upward J, Davy M, VanMeter S. A randomized, fixed-dose, dose-response study of ropinirole prolonged release in advanced Parkinson's disease. Neurodegener Dis Manag. 2017;7(1):61–72.
Mizuno Y, Yamamoto M, Kuno S, et al. Efficacy and safety of extended- versus immediate-release pramipexole in Japanese patients with advanced and L-dopa-undertreated Parkinson disease: a double-blind, randomized trial. Clin Neuropharmacol. 2012;35(4):174–81.
Mizuno Y, Nomoto M, Hasegawa K, et al. Rotigotine vs ropinirole in advanced stage Parkinson's disease: a double-blind study. Parkinsonism Relat Disord. 2014;20(12):1388–93.
Wang Y, Sun S, Zhu S, et al. The efficacy and safety of pramipexole ER versus IR in Chinese patients with Parkinson's disease: a randomized, double-blind, double-dummy, parallel-group study. Transl Neurodegener. 2014;3:11.
Zhang Z, Wang J, Zhang X, et al. The efficacy and safety of ropinirole prolonged release tablets as adjunctive therapy in Chinese subjects with advanced Parkinson's disease: a multicenter, double-blind, randomized, placebo-controlled study. Parkinsonism Relat Disord. 2013;19(11):1022–6.
Mizuno Y, Abe T, Hasegawa K, et al. Ropinirole is effective on motor function when used as an adjunct to levodopa in Parkinson's disease: STRONG study. Mov Disord. 2007;22(13):1860–5.
Stevens JW. A note on dealing with missing standard errors in meta-analyses of continuous outcome measures in WinBUGS. Pharm Stat. 2011;10(4):374–8.
Acknowledgements
Funding
Funding for this study (internal study number 206707/HO-16-17237) and the journal’s article processing charges was provided by GSK.
Medical Writing and/or Editorial Assistance
Editorial support in the form of formatting text, tables and figures and grammatical editing was provided by Jennie McLean, PhD, Fishawack Indicia Ltd, UK, and funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
James Cooper is an employee of, and holds stocks/shares in GSK. Daniel Parks is an employee of, and holds stocks/shares in GSK. Rodrigo Refoios Camejo is an employee of, and holds stocks/shares in GSK. Hongxin Zhao was an employee of GSK at the time of the study and is now employed by Synyi AI, Shanghai, China. Yi Ning was an employee of GSK at the time of the study and is now employed by Meinian Institute of Health, Beijing, China and Peking University School of Public Health, Beijing, China. Xiajun Ni was an employee of GSK at the time of the study and is now employed by Eisai China Inc. Jiangsu Province, China. Bingming Yi was an employee of GSK at the time of the study and is now employed by Vertex Pharmaceuticals, Boston MA, USA
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7857452.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, H., Ning, Y., Cooper, J. et al. Indirect Comparison of Ropinirole and Pramipexole as Levodopa Adjunctive Therapy in Advanced Parkinson’s Disease: A Systematic Review and Network Meta-Analysis. Adv Ther 36, 1252–1265 (2019). https://doi.org/10.1007/s12325-019-00938-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00938-1