Abstract
Introduction
Cutaneous adverse events (AEs) have been observed in clinical studies of daclizumab high-yield process (HYP) in relapsing-remitting multiple sclerosis (RRMS). Here, we report cutaneous AEs observed in the randomized, double-blind, active-comparator DECIDE study (ClinicalTrials.gov identifier, NCT01064401).
Methods
DECIDE was a randomized, double-blind, active-controlled phase 3 study of daclizumab HYP 150 mg subcutaneous every 4 weeks versus interferon (IFN) beta-1a 30 mcg intramuscular (IM) once weekly in RRMS. Treatment-emergent AEs were classified and recorded by investigators. Investigators also assessed the severity of each AE, and whether it met the criteria for a serious AE. Cutaneous AEs were defined as AEs coded to the Medical Dictionary for Regulatory Activities System Organ Class of skin and subcutaneous tissue disorders. The incidence, severity, onset, resolution, and management of AEs were analyzed by treatment group.
Results
Cutaneous AEs were reported in 37% of daclizumab HYP-treated patients and 19% of IFN beta-1a-treated patients. The most common investigator-reported cutaneous AEs with daclizumab HYP were rash (7%) and eczema (4%). Most patients with cutaneous AEs remained on treatment (daclizumab HYP, 81%; IM IFN beta-1a, 90%) and had events that were mild or moderate (94% and 98%) and subsequently resolved (78% and 82%). Most patients with cutaneous AEs did not require treatment with corticosteroids or were treated with topical corticosteroids (daclizumab HYP, 73%; IM IFN beta-1a, 81%). Serious cutaneous AEs were reported in 14 (2%) daclizumab HYP patients and one (<1%) IM IFN beta-1a patient.
Conclusion
There was an increased risk of cutaneous AEs with daclizumab HYP. While physicians should be aware of the potential for serious cutaneous AEs, the typical cutaneous AEs were mild-to-moderate in severity, manageable, and resolved over time.
Funding
Biogen and AbbVie Biotherapeutics Inc.
Trial registration
ClinicalTrials.gov identifier, NCT01064401.
Similar content being viewed by others
Introduction
Daclizumab high-yield process (HYP) is a humanized monoclonal antibody targeted against CD25, the interleukin 2 receptor alpha subunit [1]. Interleukin 2 is a cytokine produced by activated T cells that regulates both immune responses and the maintenance of self-tolerance [2]. Blockade of CD25 showed promising results in T cell-mediated autoimmune disorders, including in pilot studies in multiple sclerosis (MS) [3–5]. Subsequently, the efficacy and safety of daclizumab HYP have been evaluated in clinical studies in relapsing-remitting MS (RRMS) [6, 7].
In the pivotal 1-year SELECT study (ClinicalTrials.gov identifier, NCT00390221), daclizumab HYP 150 mg subcutaneous (SC) every 4 weeks significantly reduced relapses, 12-week confirmed disability progression, and brain lesions on magnetic resonance imaging (MRI) compared with placebo [6]. Daclizumab HYP also showed superior efficacy to interferon (IFN) beta-1a 30 mcg intramuscular (IM) once weekly with respect to annualized relapse rate, MRI lesion activity, and 24-week confirmed disability progression over 2–3 years of treatment in the active-comparator phase 3 DECIDE study (ClinicalTrials.gov identifier, NCT01064401) [7]. Based on the safety profile of daclizumab HYP in SELECT [6], one of the adverse events (AEs) of interest in DECIDE was cutaneous AEs. The objective of this report is to provide a description of the frequency and character of cutaneous AEs in DECIDE.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The DECIDE study protocol was approved by central and local ethics committees, and the study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki of 1964, as revised in 2008 [8]. DECIDE was registered at ClinicalTrials.gov (NCT01064401). All patients provided written informed consent before participating in the study and before photographs were used for educational purposes.
Trial Design and Patients
DECIDE was a multicenter, randomized, double-blind, double-dummy, active-controlled, phase 3 study to evaluate the efficacy and safety of daclizumab HYP versus IM IFN beta-1a [7]. The study design and patient eligibility criteria have been previously reported [7]. Briefly, patients with a confirmed diagnosis of RRMS (2005 McDonald criteria 1–4) [9] were randomized 1:1 to receive daclizumab HYP 150 mg SC every 4 weeks or IFN beta-1a 30 mcg IM once weekly for a minimum of 96 weeks up to a maximum of 144 weeks. The study ended when the last enrolled patient completed 96 weeks of treatment.
Safety Assessments
Treatment-emergent AEs and serious AEs, defined as events that started after the first dose of study drug and up to 180 days after the last dose, were monitored, classified, and recorded by investigators throughout the study. Serious AEs were followed until the event resolved, stabilized, or returned to baseline, even in patients who had completed or discontinued the study. Investigators assessed AE severity (mild, moderate, severe), whether AEs and serious AEs were or were not related to study treatment, and whether an AE met the criteria for a serious AE based on guidance in the protocol (please see Table S1 in the supplementary material for details). A serious AE was an AE that resulted in hospitalization/prolongation of hospitalization, disability/incapacity, a congenital anomaly/birth defect, or death, or in the opinion of the investigator was life-threatening. Medical history of rash, dermatitis, eczema, or psoriasis was derived from a search of patients’ medical history obtained at enrollment.
An independent central dermatologist blinded to study treatment reviewed and provided assessments of clinically significant events and the overall pattern of cutaneous AEs to the study’s data safety monitoring board. In addition, guidelines for the management of patients with cutaneous AEs were developed and provided to the study investigators, although implementation of these guidelines was not mandated by the protocol (see Appendix S1).
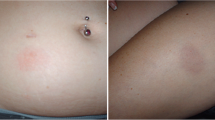
To provide a visual frame of reference, photographs of mild, moderate, and severe events in daclizumab HYP-treated patients were selected for inclusion in the manuscript from a limited subset for which patients had provided written consent for educational use.
Data Analyses
Cutaneous AEs were defined as all events coded to the Medical Dictionary for Regulatory Activities (MedDRA; version 16.1) System Organ Class of skin and SC tissue disorders. This System Organ Class does not include the Preferred Terms directly related to injection site reactions. The incidence of AEs was analyzed based on treatment group.
Results
Patients and Study Drug Exposure
DECIDE enrolled 1841 patients with RRMS; 919 were randomized and treated with daclizumab HYP and 922 were treated with IM IFN beta-1a. At baseline, patient demographic and clinical characteristics were similar between treatment groups [7]. Briefly, 68% of patients were female, 90% were white, and 41% had received prior treatment with disease-modifying therapies in each treatment group. In the daclizumab HYP and IM IFN beta-1a groups, the mean (standard deviation) age was 36.4 (9.4) and 36.2 (9.3) years, respectively. At baseline, 16% of patients in each treatment group had a medical history of a dermatological condition.
The mean (median) time on treatment was 102.0 (108.7; range 0.1–145.3) weeks and 100.5 (111.4; range 0.1–146.1) weeks in the daclizumab HYP and IM IFN beta-1a groups, representing 1797.2 and 1776.6 patient-years of treatment exposure, respectively.
Cutaneous AE Onset and Severity
Treatment-emergent cutaneous AEs were reported at generally stable rates in both groups when analyzed by 12-week intervals over 96 weeks of treatment, although the rates were consistently higher with daclizumab HYP than with IM IFN beta-1a (Fig. 1a). The cumulative incidence of cutaneous AEs was 37% in the daclizumab HYP group compared with 19% in the IM IFN beta-1a group over the course of the study. During the 6-month period after the last dose of study treatment, the incidence of cutaneous AEs remained higher in the daclizumab HYP group (21%) compared with the IM IFN beta-1a group (5%), but was similar between the groups beyond 6 months after the last dose (daclizumab HYP, <1%; IM IFN beta-1a, <1%). Cutaneous AEs were more frequently assessed by the investigator as being related to study treatment in daclizumab HYP-treated patients (15%) than in IM IFN beta-1a-treated patients (7%).
The most common cutaneous AEs (≥2% of patients in either treatment group) are listed in Table 1. Dermatitis, eczema, and rashes were among the most common investigator-reported Preferred Terms for cutaneous AEs (Table 1). There was a 1.5- to 2.0-fold higher incidence of cutaneous AEs in patients with a medical history of rash, dermatitis, eczema, or psoriasis than in those with no medical history of these conditions in both the daclizumab HYP (history: 56%, 85/152 vs no history: 34%, 259/767) and IM IFN beta-1a (history: 35%, 58/164 vs no history: 16%, 118/758) groups.
Cutaneous AEs represented 30% (43/142) and 6% (7/112) of all AE-related treatment discontinuations and 23% (15/64) and 6% (4/66) of AE-related study withdrawals in the daclizumab HYP and IM IFN beta-1a groups. Cutaneous AEs led to treatment discontinuation in a higher percentage of daclizumab HYP-(5%) versus IM IFN beta-1a-treated (<1%) patients (Table 2). Most patients with cutaneous AEs had no action taken with their study drug as a result of the event (daclizumab HYP, 80.5%; IM IFN beta-1a, 89.8%; Table 2).
The majority of patients with cutaneous AEs had events that were mild or moderate in severity (daclizumab HYP: 94%, 323/344; IM IFN beta-1a: 98%, 173/176). The most common mild and moderate cutaneous AEs (≥2% of patients in either treatment group) are listed in Table 3. Severe cutaneous AEs were more frequent in daclizumab HYP-(2%) versus IM IFN beta-1a-treated (<1%) patients (Table 3). The median (range) onset to first moderate or severe cutaneous AE was 413 (1–1121) days in the daclizumab HYP group and 311 (2–1033) days in the IM IFN beta-1a group. When evaluated by 12-week intervals, the rate of appearance of moderate or severe cutaneous AEs was steady over 96 weeks in both treatment groups (Fig. 1b); however, the overall incidence of moderate or severe cutaneous AEs was higher with daclizumab HYP than with IM IFN beta-1a over the duration of the study (see Figure S1). Based on currently available evidence, there is no clear association between cutaneous AEs and efficacy in daclizumab HYP-treated patients (data on file [Biogen]). Visual examples of mild, moderate, and severe cutaneous AEs in daclizumab HYP-treated patients are shown in Figure S2.
Resolution and Management
A total of 670 cutaneous AEs were reported in the daclizumab HYP group and 247 cutaneous AEs in the IM IFN beta-1a group. Most cutaneous AEs were reported as resolved in the daclizumab HYP (78%, 525/670) and IM IFN beta-1a (82%, 203/247) groups. In the daclizumab HYP and IM IFN beta-1a groups, 144/190 (76%) and 56/66 (85%) moderate cutaneous AEs and 18/23 (78%) and 1/3 (33%) severe cutaneous AEs were reported as resolved.
Most patients with mild cutaneous AEs (daclizumab HYP: 81%, 155/191; IM IFN beta-1a: 87%, 106/122) or moderate cutaneous AEs (daclizumab HYP: 72%, 95/132; IM IFN beta-1a: 73%, 37/51) did not require treatment with corticosteroids or were treated with topical corticosteroids (Fig. 2). In patients with severe cutaneous AEs, 81% (17/21) of daclizumab HYP-treated patients and 33% (1/3) of IM IFN beta-1a-treated patients received systemic corticosteroids (Fig. 2).
Serious Cutaneous AEs
Serious cutaneous AEs were reported in 14 (2%) daclizumab HYP-treated patients and one (<1%) IM IFN beta-1a-treated patient. In the daclizumab HYP group, dermatitis and angioedema were reported by the investigators in three and two patients, respectively; all other serious cutaneous AEs were reported in one patient each (Table 4). The serious cutaneous AE in the IM IFN beta-1a group was reported as an oral sebaceous cyst considered unrelated to study treatment in a patient with a history of cigarette smoking who was admitted to the hospital for removal of the cyst (Table 4). Among serious cutaneous AEs in the daclizumab HYP group, investigators classified ten as severe, two moderate, and two mild in severity, and three as not related to study drug (Table 4). In the daclizumab HYP group, six patients discontinued treatment due to a serious cutaneous AE but remained in the study, and two patients withdrew from the study due to a serious cutaneous AE but continued to be monitored (Table 4). All serious cutaneous AEs resolved. Patients with serious cutaneous AEs in the daclizumab HYP group were frequently treated with systemic corticosteroids (Fig. 2).
One case of drug reaction with eosinophilia and systemic symptoms (DRESS) was reported in a patient who had previously discontinued daclizumab HYP 128 days before the onset of the serious AE. The patient was treated with short tapers of betamethasone (maximum dose of 2.5–5.0 mg [equivalent to 21–42 mg of prednisone]) or betamethasone 0.5 mg (4-mg prednisone equivalent) before being hospitalized for a worsening rash. In the hospital, the patient was treated with high-dose corticosteroids and one course of plasma exchange consisting of one treatment. The event resolved. The blinded independent central dermatologist considered the event to more likely represent a grade 2 delayed-type hypersensitivity cutaneous reaction based on the available information. A broad search of the clinical database was conducted, and no additional cases were found that met the RegiSCAR criteria [10] for DRESS based on medical review of the cases.
Adjudication by Blinded Dermatologist
Based on the evaluation of cutaneous AEs in DECIDE, the central dermatologist concluded that daclizumab HYP was associated with an increased risk of cutaneous AEs. The central dermatologist reviewed and provided assessments for all cutaneous AEs considered clinically significant by the investigators. Over the course of the study, the central dermatologist reviewed case reports for 42% (284/670) of all cutaneous AEs in the daclizumab HYP group and 28% (70/247) of all cutaneous AEs in the IM IFN beta-1a group. The overall subjective impression of the central dermatologist was that cutaneous AEs in DECIDE resembled eczematous or psoriasis-like events or were typical of skin conditions commonly encountered in a dermatology clinic. In addition, there were a small number of cases that were classified as delayed-type drug hypersensitivity, most of which were mild-to-moderate in severity. In the opinion of the blinded central dermatologist, there were no cases that represented life-threatening events. There were no reported cases of Stevens–Johnson syndrome or toxic epidermal necrolysis.
Discussion
In DECIDE, daclizumab HYP was associated with a higher risk of cutaneous AEs over 2–3 years of treatment compared with IM IFN beta-1a in patients with RRMS. Relevant medical history of dermatological conditions exacerbated this risk to a similar degree in both treatment groups. Consistent with these observations, the incidence of cutaneous AEs was higher with daclizumab HYP 150 mg SC (18%) than placebo (13%) in the 1-year SELECT study [6]. Cutaneous AEs reported here are distinct from injection site reactions, which are localized and temporally linked to the injection [11].
Consistent with these findings, a recent open-label, prospective study has demonstrated a high frequency of new-onset cutaneous events associated with daclizumab HYP administration in patients with RRMS [12]. The majority of cutaneous findings in this study were consistent both clinically and histologically with an eczematous dermatitis [12]. In biopsies, cellular infiltrates in the epidermis and dermis consisted of abundant T cells, and immunohistochemistry revealed increased expression of CD56, highlighting either NK cells or CD8+ T cells amongst them [12]. The authors suggested that cutaneous events represent pharmacologic (mechanistic) immune modulation by daclizumab HYP, rather than typical drug hypersensitivity-type reactions [12]. A previous chart review study identified a case of alopecia following the development of a severe exfoliative rash in a patient treated with intravenous daclizumab (Zenapax®, Roche Laboratories, Nutley, NJ, USA) [13]. Both the rash and alopecia resolved following discontinuation of daclizumab and corticosteroid treatment [13]. In the DECIDE study, alopecia was reported in 16 (2%) patients in the daclizumab HYP group (see Table 1). All of these cases of alopecia were mild or moderate in severity; no cases of severe alopecia were reported (see Table 3).
A strength of DECIDE was the inclusion of a blinded central dermatologist, which allowed for accurate monitoring and assessment of cutaneous AEs. One limitation of this study was that very few biopsies were performed on cutaneous lesions. Results from biopsies may have provided valuable information to better understand the nature of the cutaneous AEs. In addition, photographs of cutaneous AEs were limited in number and some were of insufficient quality to be useful. Interpretation of the rates of resolution of cutaneous AEs in both treatment groups may be limited by the background rate of chronic dermatologic conditions known to occur in both the general population and in patients with MS [14, 15] and by incomplete data in patients who withdrew from the study with an ongoing non-serious AE. Because investigators were provided with cutaneous event guidelines and were made aware of this type of AE, a higher treatment rate with corticosteroids may have resulted from the distribution of these guidelines.
The potential link between the underlying mechanisms of daclizumab HYP and cutaneous AEs is unclear. Skin-resident regulatory T (Treg) cells may counteract inflammatory T cell responses, and alterations in Treg cell function in the skin have been observed in psoriasis [16]. CD25 blockade results in both expansion of CD56bright natural killer cells [17, 18] and reversible declines in the number of circulating Treg cells in patients with RRMS [19, 20]. Treg cells remaining in circulation during CD25 blockade appeared to preserve the Treg phenotype [19], although reductions in suppressive capacity have been observed [20]. As such, the relationship between reductions in Treg cells and cutaneous AEs during CD25 blockade is currently inconclusive [19, 20]. Additional work is required to determine whether compartmental differences exist in the maintenance of organ-specific self-tolerance by Treg cells [20].
Early recognition and treatment of inflammatory skin conditions offers the best chance for reversing or limiting flares of the disease [21, 22]. Dermatologic treatment guidelines recommend topical agents, including moisturizers and topical corticosteroids, for psoriasis and eczema [23, 24]. Moisturizers maintain skin hydration and can reduce itching [23]. Topical corticosteroids are recommended to decrease inflammation, and numerous formulations are available that range in relative potency (see Table S2) [23, 24]. Higher-potency topical corticosteroids are generally recommended as the initial therapy for acute flares on most body parts in adults, and lower-potency agents for maintenance therapy [23, 24]. Topical treatments, including corticosteroids, are also recommended for use with systemic treatments in more extensive eczema or psoriasis [23, 24]. Systemic corticosteroids are only recommended for acute severe exacerbations of eczema [25], and are generally contraindicated in psoriasis [26]. The overall pattern of corticosteroid treatment for cutaneous AEs in DECIDE was generally consistent with these treatment guidelines, wherein topical corticosteroids were more frequently used for mild and moderate cutaneous AEs and systemic corticosteroids for severe or serious events.
Dermatologic Perspectives of Daclizumab HYP-Related Cutaneous AEs
For the range of cutaneous AEs observed in both treatment groups in DECIDE, one must have an understanding of the overall frequency of skin diseases across world populations and the function of skin as an immunologic organ in both health and disease. Surveys have shown that eight of the most common medical problems in humans are skin conditions [27], including eczema, which was a frequent investigator-reported diagnosis in DECIDE. Atopic eczema is also a common skin condition in up to 30% of children, where the majority outgrow eczema by early adulthood but there is also an increasing frequency of adult-onset atopic eczema across world populations [21, 28, 29]. Hence, one perspective is that a large range of common skin conditions are also seen in the DECIDE study population. Within the spectrum of cutaneous AEs in DECIDE, there was a large representation of eczema and eczema-spectrum conditions (i.e., allergic dermatitis, eczema, nummular dermatitis, and hand dermatitis) [29], which may be classified as inflammatory skin diseases. Among patients with cutaneous AEs, more than one-third (133/344 [39%]) had investigator-reported AEs coded to the MedDRA High Level Term dermatitis and eczema (see Table 1). Although eczematous events are a nuisance, they typically tend to be reversible and do not result in skin damage or organ failure even if present for extended periods [26, 30]. Chronic rashes are likely driven by the interplay between genetic predisposition, immune response, and environmental factors (e.g., tendency for re-emergence of eczema in humans) [22]. Importantly, daclizumab HYP did not lead to cutaneous AEs that resulted in loss of skin structure or function. Most cutaneous AEs during daclizumab HYP treatment were not allergic reactions. However, a small number of drug-type hypersensitivity reactions were identified; these reactions need to be distinguished from inflammatory diseases, as it may be necessary to discontinue drugs that drive drug-type hypersensitivity reactions. Overall, early diagnosis and treatment of cutaneous AEs following established dermatologic management guidelines offer the best opportunity for resolution.
We speculate that many of the inflammatory skin conditions noted in DECIDE relate mechanistically to the function of skin as an immune organ and to mechanistic effects on Treg cells or other regulatory immune mechanisms. Skin contains resident dendritic antigen-presenting cells and skin-homing resident memory T cells; therefore, immune reactions confined only to the skin are possible [16, 31]. The range of commonly encountered skin diseases, such as atopic eczema and psoriasis, represent excessive activation of T cells in the skin, and skin inflammation is produced by effects of T cell-derived cytokines on resident skin cells, in part enabled by the reduced function of Treg cells [32]. Targeted removal of cutaneous Treg cells in model systems produces skin inflammation and excess production of cytokines that typify atopic eczema and related diseases [33]. Hence, the decrease in Treg cells noted in circulating T cells during daclizumab HYP treatment [19, 20] may well extend to diminished function of these cells with the cutaneous compartment, leading to unmasking or activation of resident T cell populations that mediate psoriasis or eczema-spectrum conditions.
Conclusion
In summary, cutaneous AEs in daclizumab HYP-treated patients were predominantly mild-to-moderate in severity, treated with topical corticosteroids or did not require treatment with a corticosteroid, and resolved. More severe or serious cutaneous AEs frequently required treatment with systemic corticosteroids. Some patients received multiple courses of corticosteroids when the cutaneous AE was of longer duration. Most importantly, the cutaneous AEs observed with daclizumab HYP were manageable with standard interventions and, even in the smaller number of serious cases, did not lead to any life-threatening conditions.
References
Wiendl H, Gross CC. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol. 2013;9:394–404.
Waldmann TA. The IL-2/IL-15 receptor systems: targets for immunotherapy. J Clin Immunol. 2002;22:51–6.
Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci USA. 1999;96:7462–6.
Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon ß. Proc Natl Acad Sci USA. 2004;101:8705–8.
Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol. 2004;56:864–7.
Gold R, Giovannoni G, Selmaj K, et al; SELECT study investigators. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:2167–75.
Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373:1418–28.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 1 Nov 2014.
Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–6.
Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80.
McEwan L, Brown J, Poirier J, et al. Best practices in skin care for the multiple sclerosis patient receiving injectable therapies. Int J MS Care. 2010;12:177–89.
Cortese I, Ohayon J, Fenton K, et al. Cutaneous adverse events in multiple sclerosis patients treated with daclizumab. Neurology. 2016;86:847–55.
Oh J, Saidha S, Cortese I, et al. Daclizumab-induced adverse events in multiple organ systems in multiple sclerosis. Neurology. 2014;82:984–8.
Edwards LJ, Constantinescu CS. A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler. 2004;10:575–81.
Ramagopalan SV, Dyment DA, Valdar W, et al; Canadian Collaborative Study Group. Autoimmune disease in families with multiple sclerosis: a population-based study. Lancet Neurol. 2007;6:604–10.
Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–70.
Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5941–6.
Elkins J, Sheridan J, Amaravadi L, et al. CD56bright natural killer cells and response to daclizumab HYP in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2015;2:e65.
Huss DJ, Mehta DS, Sharma A, et al. In vivo maintenance of human regulatory T cells during CD25 blockade. J Immunol. 2015;194:84–92.
Oh U, Blevins G, Griffith C, et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol. 2009;66:471–9.
Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94.
Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–79.
Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116–32.
Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–59.
Sidbury R, Davis DM, Cohen DE, et al; American Academy of Dermatology. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327–49.
Gottlieb AB. Therapeutic options in the treatment of psoriasis and atopic dermatitis. J Am Acad Dermatol. 2005;53(suppl 1):S3–16.
Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527–34.
Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593–600.
Hanifin JM, Reed ML; Eczema Prevalence and Impact Working Group. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82–91.
Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445:866–73.
Girardi M. Cutaneous perspectives on adaptive immunity. Clin Rev Allergy Immunol. 2007;33:4–14.
Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–32.
Freyschmidt EJ, Mathias CB, Diaz N, et al. Skin inflammation arising from cutaneous regulatory T cell deficiency leads to impaired viral immune responses. J Immunol. 2010;185:1295–302.
Acknowledgments
This study and the article processing charges for this publication were funded by Biogen and AbbVie Biotherapeutics Inc. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Biogen and AbbVie Biotherapeutics Inc. provided funding for medical writing support in the development of this paper; Alison Gagnon, Ph.D., from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Kristen DeYoung from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. Biogen and AbbVie Biotherapeutics Inc. reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper, and provided their final approval of all content. Some of these data have been presented previously as an abstract and poster presentation at the 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 7–10, 2015; Barcelona, Spain.
Disclosures
J. G. Krueger has served as a consultant for Biogen and reviews and adjudicates cutaneous adverse events in Biogen- and AbbVie Biotherapeutics Inc.-sponsored clinical studies, including DECIDE. L. Kircik has received consulting honoraria from Biogen; and travel support from and has served as a consultant, speaker, or investigator for Abbott, Amgen, Celgene, Genentech, Janssen, Novartis, Pfizer, Sandoz, and Valeant. F. Hougeir serves as a consultant for Biogen and AbbVie Biotherapeutics Inc. A. Friedman serves as a consultant for Amgen, Aveeno, Biogen, Galderma, Intraderm, L’Oréal, MicroCures, Nano BioMed, Nerium Biotechnology, Oculus, PHD Skin Care, Pfizer, Salvona, and Valeant. N. Lucas, W. Castro-Borrero, P. McCroskery, and J. Elkins are full-time employees of and hold stock/stock options in Biogen. S. J. Greenberg is a full-time employee of and holds stock/stock options in AbbVie. X. You and M. Sweetser were full-time employees of Biogen at the time of this analysis and hold stock/stock options in Biogen. X. You is currently a full-time employee of Vertex Pharmaceuticals, and M. Sweetser is currently a full-time employee of Alnylam Pharmaceuticals, Inc.
Compliance with Ethics Guidelines
The DECIDE study protocol was approved by central and local ethics committees, and the study was conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice and the Declaration of Helsinki of 1964, as revised in 2008. DECIDE was registered at ClinicalTrials.gov (NCT01064401). All patients provided written informed consent before participating in the study and before photographs were used for educational purposes.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/85D4F06061A0B6E0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krueger, J.G., Kircik, L., Hougeir, F. et al. Cutaneous Adverse Events in the Randomized, Double-Blind, Active-Comparator DECIDE Study of Daclizumab High-Yield Process Versus Intramuscular Interferon Beta-1a in Relapsing-Remitting Multiple Sclerosis. Adv Ther 33, 1231–1245 (2016). https://doi.org/10.1007/s12325-016-0353-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-016-0353-2