Abstract
Introduction
The aim of the present study was to compare the confocal and clinical features of newly diagnosed glaucoma patients receiving unpreserved prostaglandins (tafluprost) versus preserved prostaglandins (latanoprost).
Materials and Methods
40 patients were randomized to tafluprost 0.0015% (20 patients; 32 eyes) or latanoprost 0.005% + benzalkonium chloride 0.02% (20 patients; 35 eyes) once daily for 1 year. Inclusion criteria were new glaucoma diagnosis, and no ocular treatments for 6 months before the study. Patients were evaluated at baseline and every 3 months with a complete ophthalmologic evaluation, Schirmer’s test, break-up time test, confocal microscopy of the central cornea, and measurement of intraocular pressure (IOP). Investigators were masked to treatment. Both eyes were analyzed if they fulfilled inclusion criteria. Treatments and changes between follow-up and baseline were compared by analysis of variance (ANOVA), t test and Chi-square test.
Results
At baseline, the two groups had similar age, ocular surface and confocal findings; keratocyte activation was present in 40%, branching pattern in 85%, and beading in 75%, with no inter-group differences. At follow-up, no significant clinical changes were detected, apart from a drop of IOP by 3.6–4.2 mmHg in the two groups (p < 0.001, with no difference between treatments). Despite inter-treatment ANOVA for confocal microscopy being negative, subtle changes were present. During follow-up, all eyes without nerve branching pattern at baseline progressively developed it when treated with latanoprost, whereas no change occurred using tafluprost treatment (p = 0.05). None of the eyes without beading at baseline developed it at the end of the study in the tafluprost group, whereas beading did occur in 75% of patients treated with latanoprost (p = 0.05). Both treatments were associated with increased keratocyte activation at follow-up; the change from baseline was statistically significant after month 3 with latanoprost (p = 0.02) and after month 6 with tafluprost (p = 0.04).
Conclusions
The two study treatments had similar clinical effects, but tafluprost had a more favorable profile for some confocal parameters of the cornea.
Funding
Merck Sharp & Dohme International.
Similar content being viewed by others
Introduction
The beneficial effect of intraocular pressure (IOP) lowering treatments to reduce glaucoma progression has been demonstrated by a number of multicenter, randomized studies [1–4]. On the other hand, more recent studies have also shown the detrimental effects of medical treatments for glaucoma on the ocular surface [5–11]. It has been shown that prostaglandin analogs have inflammatory effects [5–9, 11], yet the vast majority of side effects are due to preservatives, in particular benzalkonium chloride (BAK), which is the most toxic and most used of ophthalmic preservatives [5–11]. BAK effects are dose dependent [7–12], and this is relevant considering that most glaucoma patients receive more than one IOP-lowering treatment [4]. Chronic BAK exposure is also associated with reduced efficacy of glaucoma surgery [13]. As a consequence, preservative-free treatments are preferable for glaucoma, as for all chronic eye diseases [14].
Confocal microscopy is a recent technique which enables ophthalmologists to detect subtle inflammatory and toxic changes of the ocular surface [15]. By means of confocal microscopy, BAK has been shown to reduce the density of conjunctival goblet cells [16, 17], of conjunctival and corneal epithelial cells [17], and to deteriorate the normal characteristics of corneal nerves [18–20].
Still, timing of occurrence of ocular surface changes when starting IOP-lowering treatments is an unexplored issue. Tafluprost is the most recent unpreserved prostaglandin analog introduced in clinical practice and it is characterized by the absence of BAK.
To the best of the author’s knowledge, this is the first study to investigate and compare, from both clinical and confocal viewpoints, the effects of preserved and unpreserved prostaglandin analogs in newly diagnosed glaucoma patients with normal ocular surface.
Materials and Methods
A randomized, masked, prospective study was carried out to test the primary hypothesis that treatment with preserved prostaglandins induces confocal changes of the cornea (both stromal inflammation and toxic damage to the sub-basal nerves) and that these anatomical changes would induce clinical changes, as detected during a general ophthalmic examination.
Inclusion Criteria
Inclusion criteria for the present study were: diagnosis of ocular hypertension (OH), primary open-angle glaucoma (POAG), pseudoexfoliative glaucoma or normal tension glaucoma, according to the definitions of the European Glaucoma Society Guidelines [21]; no previous treatments to reduce IOP and no treatment with any BAK-preserved eye drop for at least 6 months before the study; no fluorescein staining at baseline and no observable signs of ocular surface disease.
Exclusion Criteria
Exclusion criteria for the present study were: unwillingness to sign informed consent; aged <18 years; any ocular condition that was of safety concern or interfering with the study results; any ocular condition requiring the use of eye drops during follow-up (i.e., dry eye); closed/barely open anterior chamber angles or history of acute angle closure; ocular surgery or argon laser trabeculoplasty within the last year; ocular inflammation/infection occurring within 3 months prior to pre-trial visit; presence of the following ocular conditions: dry eye, moderate–severe blepharitis, Rosacea, Sjogren syndrome, pterygium or use of contact lens(es); hypersensitivity to BAK or to any other component of the trial drug solutions; any corneal pathology; diabetes at any stage; other abnormal condition or symptom preventing the patient from entering the trial (need for more than 1 IOP-lowering treatment), according to the investigator’s judgment; refractive surgery patients; women who were pregnant, of childbearing potential and not using adequate contraception or nursing; inability to adhere to treatment/visit plan.
Clinical Plan
The study protocol comprised 5 visits (performed at Eye Clinic of San Paolo Hospital, Milan, Italy): Baseline, Month 3, Month 6, Month 9 and Month 12.
At baseline, a clinical evaluator performed a complete ophthalmologic evaluation to confirm diagnosis. The following examinations were done in the following sequence: anterior segment examination, Schirmer’s test and break-up time test. Thereafter, a confocal evaluator performed confocal microscopy of the central cornea. Finally, contact measurements were carried out in the following order: IOP, pachymetry and gonioscopy. A 15-min interval between two consecutive tests was observed.
A study coordinator recorded medical history and then randomized patients into two groups: one group to receive unpreserved (tafluprost 0.0015%, Saflutan®, Santen Pharmaceutical, Osaka, Japan) and one group to receive preserved prostaglandins (latanoprost 0.005% + BAK 0.02%, Xalatan®, Pfizer S.r.L., Latina, Italy) once daily to both eyes (randomization of 1:1, by means of a list of random numbers). Being patients treated to both eyes, a control group was not available. During the study, patients were instructed not to use any other topical treatment other than the study medication. The confocal and the clinical investigators were masked to treatment.
Confocal and clinical examinations, as described above, were repeated at months 3, 6, 9 and 12.
Adherence to treatment, medical history, and side effects were checked by study coordinator at follow-up visits. Adverse effects were recorded. Symptoms were evaluated by means of comparison of ophthalmic medications for tolerability (COMTOL) questionnaire [22].
Corneal Confocal Biomicroscopy
The second version of Heidelberg Retina Tomograph (Heidelberg Engineering, Heidelberg, Germany) is endowed with a lens system called the [Rostock Cornea Module (RCM)], and allows an in vivo confocal study of the ocular surface. The laser source used in the RCM is a diode laser with a wavelength of 670 nm. The acquired two-dimensional images have a definition of 384 × 384 pixels over an area of 400 µm × 400 µm with lateral digital resolution of 1 µm/pixel and a depth resolution of 2 µm/pixel.
After administration of one drop of 0.4% oxybuprocaine and one drop of a lubricant gel (0.2% carbomer), the patient was asked to fixate on a small, bright, red light as the examination was performed in the contralateral eye. Correct alignment and contact with the cornea were monitored using the images captured by a camera tangential to the eye. The distance from the cornea to the microscope was kept stable using a single-use contact element in sterile packaging, (TomoCap, Heidelberg Engineering, Heidelberg, Germany). The examination took about 7 min per eye; 5 images of each cornea layer and of the sub-basal layer were collected, both in central area. The highest resolution images taken of the different layers were considered for the analysis.
Test–retest variability of confocal microscopy of the central cornea was tested at the beginning of the study using the following method. 5 eyes of 5 volunteers were tested 3 times each: twice during the same day (at 9 a.m. and at 11 a.m.) and once the day after (at 9 a.m.). The confocal operator evaluated these images and found an agreement of 80% or more for all parameters.
Sample Size Calculation
Given the paucity of information available on the effects of treatments with BAK-free prostaglandin on the ocular surface studied by confocal imaging, sample size calculation for this pilot study may be imprecise. The outcome of the study was corneal inflammation at confocal microscopy (defined as activation of anterior stroma, changes of nerve morphology, increase of dendritic cells). If a worth-detecting difference of 40% between the two groups is assumed, the presence of subclinical inflammation in 30% of normal cases, a one-tailed distribution in favor of the BAK-free arm of the study, α = 0.05, β = 0.2, a sample of 20 eyes would be necessary [20, 23, 24]. It was decided to overpower the study including all treated eyes (a control group was absent in any case, being patients treated to both eyes), and this gave a study power of nearly 90%.
Statistical Analysis
All available data were analyzed (i.e., all eyes receiving study product were analyzed). The dataset was analyzed by means of linear and generalized, mixed-effect models of analysis of variance (ANOVA), with a post hoc test. In case of multiple comparisons, t test and Chi-square tests with Bonferroni–Holm correction were used. R open-access software was used (version 3.1.3, R foundation for statistical computing, Vienna, Austria).
Compliance with Ethics
This present study was performed at the Eye Clinic, Department of Medicine, Surgery and Odontoiatry, San Paolo Hospital, University of Milan, Italy.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (University of Milan, Italy) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
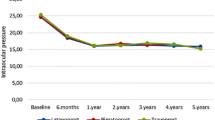
Forty consecutive patients with new diagnosis of glaucoma or ocular hypertension were enrolled between January and July 2013. The study included 32 and 35 eyes in the tafluprost and latanoprost groups, respectively. Demographic characteristics of the study population and main study results are given in Tables 1, 2 and 3. The two groups had similar age and ocular surface and confocal findings at baseline (Figs. 1, 2). At the beginning of the study, activation of anterior stromal keratocytes was present in 40% of total patients (28% and 50% of subjects in latanoprost and tafluprost groups, respectively, p = 0.08); branching pattern was present in about 85% of patients, and beading in 75% of cases.
Confocal images of a patient treated with tafluprost. a Sub-basal plexus at baseline. b Sub-basal plexus at month 12: no relevant changes of density, length, morphology are shown. c Anterior stroma at baseline; no keratocyte activation is present. d Anterior stroma at month 12: no changes are shown; keratocyte activation is absent
Confocal images of a patient treated with latanoprost. a Sub-basal plexus at baseline. b Anterior stroma at baseline; no keratocyte activation is present. c Sub-basal plexus at month 12: disruption of normal nerve structure is shown: branching and beading are present, and nerve is tortuous; density is overall conserved. d Anterior stroma at month 12 showing keratocyte activation
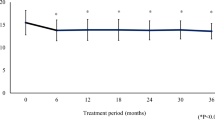
During a 1-year interval from treatment beginning, no significant clinical changes were detected, apart from a drop of IOP of 3.6–4.2 mmHg in the two groups (p < 0.001, with no statistically significant difference between treatments; ANOVA).
Confocal microscopy was similar between groups and between time points when analyzed by ANOVA. Yet, subtle changes occurring on the morphology of the cornea were shown at follow-up. All patients without branching pattern of sub-basal nerves at baseline progressively (from 9 to 12 months) developed this pattern when treated with latanoprost, whereas no change occurred at follow-up in subjects treated with tafluprost (p = 0.04, month 12). None of the patients without beading at baseline developed beading at the end of the study in tafluprost group, whereas this occurred in 6/8 (75%) patients treated with latanoprost (p = 0.05).
Both treatments were associated with an increase of activation of anterior stromal keratocytes at follow-up; the change from baseline was statistically significant 3 months after starting treatment with latanoprost (p = 0.02) and 6 months after tafluprost (p = 0.04).
A small and not significant increase of dendritic cells density occurred over time, with no difference between treatments.
No significant side effects were detected with any treatment during the study. No significant changes of symptoms were found, as evaluated by COMTOL scale, at follow-up in the two groups. Adherence to treatment was high (96%), and no study discontinuation occurred.
Discussion
This paper explored the effects of tafluprost and latanoprost on a population of newly diagnosed POAG and OH with normal ocular surface, and the two treatments were found to have the same IOP-lowering effect and clinical tolerability over 1 year of follow-up, thus confirming previous findings [25–27].
One novelty of the present study is that by means of a parallel randomization, prospective and masked design, the two treatments were also compared using confocal microscopy. Using this method, it was shown that a subgroup of otherwise normal subjects at baseline have subclinical corneal patterns (activation of anterior stromal keratocytes, nerve beading and branching). The number of cases with activation of keratocytes increased over time, thus confirming previous findings on the pro-inflammatory effect of prostaglandin analogs (regardless of BAK) [23]. Of the changes occurring during follow-up on sub-basal nerves, beading and branching were significantly lower in patients receiving tafluprost. Another paper recently compared the corneal confocal findings of the two treatments using a non-randomized design, and showed that tafluprost has a favorable safety profile [24].
The main difference between the two study treatments is the absence of BAK in tafluprost formulation. BAK has been used for decades on nearly all ophthalmic formulations with an overall low percentage of serious side effects [28], even if recent studies demonstrated that BAK frequently causes relevant changes on the ocular surface, particularly when inspected by confocal microscopy [28].
Little is known on the timing of occurrence of ocular surface changes when starting IOP-lowering treatments; in the present study it was shown that keratocyte activation (which was present at baseline in about one-third of eyes) increases immediately after the treatment is started and it tends to increase over time, whereas morphological changes of the nerves are present only after 9–12 months.
Most of the corneal changes found in confocal studies on patients with glaucoma have been attributed to BAK. In particular, BAK has a dose-dependent apoptotic action [29] which has been shown to disrupt the epithelial barrier of both conjunctiva [16, 30] and cornea [11]; at ultrastructural levels, BAK induces a massive reduction of goblet cells [16, 30] and an anatomical disruption of corneal glycocalyx and microvilli [11]. In the most severe cases, deeper layers of the ocular surface can also be involved by BAK exposure: conjunctival fibrosis and keratinization have been reported [31]. Most recently, BAK exposure has been associated also with anterior chamber inflammation [32].
From the literature, the use of BAK-free treatments is preferable in all cases [16, 18, 30, 33, 34]. Studies comparing BAK and BAK-free treatments for glaucoma showed the superiority of BAK-free treatments on clinical findings [33, 34] and, by means of confocal microscopy, conjunctival [16, 30] and corneal [18] findings. The non-randomized, cross-sectional paper by Martone et al. [18] was one of the first to suggest that patients receiving unpreserved treatments for glaucoma have confocal findings more similar to controls than to patients treated with BAK-preserved eye drops.
Regardless of the exposure to BAK, it has been suggested that stromal activation may be facilitated by the pro-inflammatory activity of prostaglandin analogs [23]. Even if other studies found that activation may be similar for beta-blockers and prostaglandins [18, 20], the data seem to support the effect of the drug itself on the keratocyte activity.
The beneficial effect of switching from preserved to unpreserved prostaglandin treatment has been explored by a recent study which showed, over a 1-year period, an increase in epithelial and nerve densities, a reduction of keratocyte activation, a reduction of bead-like formations and nerve tortuosity [25]. Despite these premises, the present study seems to indicate that such findings may not be clinically relevant for newly diagnosed glaucoma patients, without ocular surface disease, receiving low doses of BAK (i.e., monotherapy) for a short period of time. Clinical data and symptoms, in fact, overlapped in the two study groups at all visits. The confocal difference of the two treatments may gain relevance in patients with longer follow-up, with concomitant ocular surface disease, or exposure to higher BAK concentrations due to concomitant use of preserved eye drops. These factors were outside the scope of the study, but these patients will have continued follow-ups to detect possible future clinical and confocal changes.
Readers should be aware that this study reflects the limits of confocal microscopy, i.e., subjectivity and limited repeatability. The area investigated by this device is also very small and may be not representative of the whole cornea. The data are comparable to those available in literature for corneal confocal microscopy of normal patients, with the exception of dendritic cells, which were lower in the present study sample than in literature (although Zhivov et al. [35]. suggested that dendritic cell density in normal subjects may range from 0 to 64/mm2). In general, data on confocal microscopy have a large span of normality, as shown in Table 4 [35–45]. Moreover, the discrimination between normal and abnormal findings at confocal investigation is not always univocal; for example, the role played by branching, tortuosity or abnormally high or abnormally low reflectivity is debated [16, 18–20].
Due to the paucity of data on confocal microscopy in newly treated glaucoma patients, sample size assumptions were approximate; the inclusion of all available eyes in analysis increased the statistical power of the study but could also limit its validity. Nevertheless, this paper has the merit of a randomized, double-blinded design; the confocal evaluators were blinded to the characteristics of the patients and evaluated images in a blinded fashion.
Conclusion
In conclusion, the present study found out that the low daily exposure to BAK of patients treated with latanoprost may facilitate the development of confocal changes of the cornea, which occurred less frequently on patients treated with tafluprost. Activation of anterior stromal keratocytes was present at baseline in one-third of cases and increased at follow-up, probably due to the pro-inflammatory activity of prostaglandin analogs. From a clinical viewpoint, the two treatments had similar IOP-lowering effect and tolerability.
References
AGIS Investigators. Advanced Glaucoma Intervention Study (AGIS), 7: the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40.
Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122:813–20.
Leske MC, Heijl A, Hussein M, Early Manifest Glaucoma Trial Group, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56.
Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53.
Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115:109–15.
Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2013;23:47–54.
Fechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–21.
Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–6.
Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–5.
Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120:2241–8.
Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6.
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1–9):e2.
Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma. 2013;22:730–5.
Stalmans I, Sunaric Mégevand G, Cordeiro MF, Hommer A, Rossetti L, Goñi F, Heijl A, Bron A. Preservative-free treatment in glaucoma: who, when, and why. Eur J Ophthalmol. 2013;23:518–25.
Mustonen RK, McDonald MB, Srivannaboon S, Tan AL, Doubrava MW, Kim CK. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea. 1998;17:485–92.
Frezzotti P, Fogagnolo P, Haka G, et al. In vivo confocal microscopy of conjunctiva in preservative-free timolol 0.1% gel formulation therapy for glaucoma. Acta Ophthalmol. 2014;92(2):e133–40.
Mastropasqua L, Agnifili L, Fasanella V, et al. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013;91(5):e397–405.
Martone G, Frezzotti P, Tosi GM, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147(725–735):e1.
Baratz KH, Nau CB, Winter EJ, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25:1046–52.
Ranno S, Fogagnolo P, Rossetti L, Orzalesi N, Nucci P. Changes in corneal parameters at confocal microscopy in treated glaucoma patients. Clin Ophthalmol. 2011;5:1037–42.
European Glaucoma Society. Terminology and guidelines for glaucoma. 4th ed. Dogma Editor: Savona. 2008.
Barber BL, Strahlman ER, Laibovitz R, Guess HA, Reines SA. Validation of a questionnaire for comparing the tolerability of ophthalmic medications. Ophthalmology. 1997;104:334–42.
Bergonzi C, Giani A, Blini M, Marchi S, Luccarelli S, Staurenghi G. Evaluation of prostaglandin analogue effects on corneal keratocyte density using scanning laser confocal microscopy. J Glaucoma. 2010;19:617–21.
Rossi GC, Blini M, Scudeller L, et al. Effect of preservative-free tafluprost on keratocytes, sub-basal nerves, and endothelium: a single-blind one-year confocal study on naïve or treated glaucoma and hypertensive patients versus a control group. J Ocul Pharmacol Ther. 2013;29:821–5.
Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013;97:1510–5.
Traverso CE, Ropo A, Papadia M, Uusitalo H. A phase II study on the duration and stability of the intraocular pressure-lowering effect and tolerability of Tafluprost compared with latanoprost. J Ocul Pharmacol Ther. 2010;26:97–104.
Uusitalo H, Pillunat LE, Ropo A, Phase III Study Investigators. Efficacy and safety of tafluprost 0.0015% versus latanoprost 0.005% eye drops in open-angle glaucoma and ocular hypertension: 24-month results of a randomized, double-masked phase III study. Acta Ophthalmol. 2010;88:12–9.
Tressler CS, Beatty R, Lemp MA. Preservative use in topical glaucoma medications. Ocul Surf. 2011;9:140–58.
De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of benzalkonium chloride on growth and survival of Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:619–30.
Ciancaglini M, Carpineto P, Agnifili L, et al. An in vivo confocal microscopy and impression cytology analysis of preserved and unpreserved levobunolol-induced conjunctival changes. Eur J Ophthalmol. 2008;18:400–7.
Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–34.
Stevens AM, Kestelyn PA, De Bacquer D, Kestelyn PG. Benzalkonium chloride induces anterior chamber inflammation in previously untreated patients with ocular hypertension as measured by flare meter: a randomized clinical trial. Acta Ophthalmol. 2012;90:e221–4.
Iester M, Telani S, Frezzotti P, et al. Ocular surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J Ocul Pharmacol Ther. 2014;30(6):476–81.
Kitazawa Y, Smith P, Sasaki N, Kotake S, Bae K, Iwamoto Y. Travoprost 0.004%/timolol 0.5%-fixed combination with and without benzalkonium chloride: a prospective, randomized, doubled-masked comparison of safety and efficacy. Eye. 2011;25:1161–9.
Zhivov A, Stave J, Vollmar B, Guthoff R. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243:1056–61.
Patel DV, Ku JY, Johnson R, McGhee CN. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye. 2009;23(3):586–92.
Ceresara G, Fogagnolo P, De Cillà S, et al. Corneal involvement in Crohn’s disease: an in vivo confocal microscopy study. Cornea. 2011;30:136–42.
Hu Y, Matsumoto Y, Adan ES, et al. Corneal in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Ophthalmology. 2008;115:2004–12.
Eckard A, Stave J, Guthoff RF. In vivo investigations of the corneal epithelium with the confocal Rostock laser scanning microscope (RLSM). Cornea. 2006;25:127–31.
De Cillà S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50:5155–8.
Bucher F, Adler W, Lehmann HC, et al. Corneal nerve alterations in different stages of Fuchs’ endothelial corneal dystrophy: an in vivo confocal microscopy study. Graefes Arch Clin Exp Ophthalmol. 2014;252:1119–26.
Kurbanyan K, Hoesl LM, Schrems WA, Hamrah P. Corneal nerve alterations in acute Acanthamoeba and fungal keratitis: an in vivo confocal microscopy study. Eye. 2012;26:126–32.
Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28:1253–60.
Lin H, Li W, Dong N, et al. Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci. 2010;51:122–8.
Salvetat ML, Zeppieri M, Miani F, Parisi L, Felletti M, Brusini P. Comparison between laser scanning in vivo confocal microscopy and noncontact specular microscopy in assessing corneal endothelial cell density and central corneal thickness. Cornea. 2011;30:754–9.
Acknowledgments
The paper was supported by the unrestricted grant # 00111760 by Merck Sharp & Dohme International. Article processing charges and the open access fee were supported by Santen Ltd. Registration number: NCT01433900 (at www.clinicaltrials.gov). All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis. The Authors are grateful to Giovanni Montesano, MD, Università degli Studi di Milano, Milan, Italy for his help on statistical analysis of the results of the study.
Conflict of interest
Paolo Fogagnolo received a speaker honorarium from Merck Sharp & Dohme International.
Luca Rossetti received a speaker honorarium from Merck Sharp & Dohme International.
Angelica Dipinto, Elisa Vanzulli, Emanuele Maggiolo, Stefano De Cilla’ and Alessandro Autelitano declare that they have no conflict of interest.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ethics Committee of the University of Milan, Italy) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: clinicaltrials.gov # NCT01433900.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fogagnolo, P., Dipinto, A., Vanzulli, E. et al. A 1-Year Randomized Study of the Clinical and Confocal Effects of Tafluprost and Latanoprost in Newly Diagnosed Glaucoma Patients. Adv Ther 32, 356–369 (2015). https://doi.org/10.1007/s12325-015-0205-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0205-5