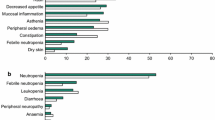
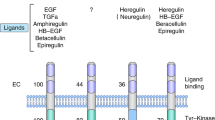
Abstract
Introduction
Targeting the human epidermal growth factor receptor (HER) family of tyrosine kinase receptors has proven to be effective as a therapeutic strategy for HER type 2 (HER2)-positive breast cancer. Since resistance to trastuzumab occurs relatively frequently, particularly in the metastatic setting, novel anti-HER2 targeted therapies with complementary and/or synergistic mechanisms of action have been under development. Pertuzumab, a HER2-targeted monoclonal antibody that prevents HER2 dimerisation, is the first of a class of promising targeted agents for the treatment of HER2-positive breast cancer.
Methods
A review of the biomedical literature published prior to February 2013 was conducted in English using PubMed. ClinicalTrials.gov was searched for appropriate clinical trials. The search terms used included breast neoplasm, pertuzumab, dimerisation, and HER2-positive. Abstracts of studies presented at the ASCO and ESMO Annual Meetings, and San Antonio Breast Cancer Symposium were also included.
Results
Pertuzumab represents a novel anti-HER2 targeted therapy for HER2-positive breast cancers. In this article, we describe the mechanism of action of pertuzumab, as well as its drug development process and preclinical testing results. Based on the results of ancillary studies, dual inhibition using pertuzumab and trastuzumab was shown to be effective for the management of HER2-positive metastatic breast cancers pre-treated with trastuzumab-based therapy. For the first-line setting, the combination of both pertuzumab and trastuzumab with docetaxel (CLEOPATRA trial; clinical evaluation of pertuzumab and trastuzumab) has changed the paradigm of patient management.
Conclusion
Pertuzumab provided a more comprehensive inhibition of HER2-driven signalling pathways. When administered together with trastuzumab, pertuzumab represent a significant advancement for the treatment of HER2-positive metastatic breast cancer patients.

Similar content being viewed by others
References
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51.
Yarden Y, Sliwkowski MX. Untangling the erbb signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37.
Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8.
Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99.
Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol. 2003;14:1346–63.
Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-her-2 therapy and personalized medicine. Oncologist. 2009;14:320–68.
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28:92–8.
Nahta R, Esteva FJ. Her2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215.
Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of her2-positive metastatic breast cancer: pivotal trials. Oncology. 2001;61(Suppl 2):14–21.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the her-2/neu oncogene. Science. 1987;235:177–82.
Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer trial. J Clin Oncol. 2009;27:5685–92.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in her2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in her2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Marty M, Cognetti F, Maraninchi D, et al. Randomized phase ii trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74.
Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of her2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16–32.
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for her2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43.
Baselga J, Gelmon KA, Verma S, et al. Phase ii trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–44.
Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for her2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:1594–600.
Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible erbb receptor tyrosine kinase inhibitor, in patients with advanced erbb2-positive breast cancer. J Clin Oncol. 2010;28:1301–7.
De Mattos-Arruda L, Cortes J. Breast cancer and HSP90 inhibitors: is there a role beyond the her2-positive subtype? Breast. 2012;21:604–7.
De Mattos-Arruda L, Cortes J. Advances in first-line treatment for patients with her-2+ metastatic breast cancer. Oncologist. 2012;17:631–44.
Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into erbb signaling from the structure of the erbb2-pertuzumab complex. Cancer Cell. 2004;5:317–28.
Adams CW, Allison DE, Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic her dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–27.
Baselga J. A new anti-erbb2 strategy in the treatment of cancer: prevention of ligand-dependent erbb2 receptor heterodimerization. Cancer Cell. 2002;2:93–5.
Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated erbb2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37.
Sergina NV, Rausch M, Wang D, et al. Escape from her-family tyrosine kinase inhibitor therapy by the kinase-inactive her3. Nature. 2007;445:437–41.
Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70.
Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA. Nonclinical studies addressing the mechanism of action of trastuzumab (herceptin). Semin Oncol. 1999;26:60–70.
Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on her2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6.
Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–6.
Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel her dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–43.
Attard G, Kitzen J, Blagden SP, et al. A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumours. Br J Cancer. 2007;97:1338–43.
Albanell J, Montagut C, Jones ET, et al. A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhumab 2c4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res. 2008;14:2726–31.
Yamamoto N, Yamada Y, Fujiwara Y, et al. Phase I and pharmacokinetic study of her2-targeted rhumab 2c4 (pertuzumab, ro4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2009;39:260–6.
Ng CM, Lum BL, Gimenez V, Kelsey S, Allison D. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res. 2006;23:1275–84.
Gianni L, Llado A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–7.
Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–6.
Swain SM, Kim S-B, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–71.
Miles D, Baselga J, Amadori D, et al. Pertuzumab (p) in combination with trastuzumab (T) and docetaxel (D) in elderly patients with HER2-positive metastatic breast cancer in the Cleopatra study. Cancer Res. 2012;72(24 Suppl):P5-18-01 (Abstract).
Baselga J, Cortés J, Im S-A, et al. Biomarker analyses in Cleopatra: a phase iii, placebo-controlled study of pertuzumab in her2-positive, first-line metastatic breast cancer (mbc). Cancer Res. 2012;72(24 Suppl):S5-1 2012 (Abstract).
Datko F, D’Andrea G, Dickler M, et al. Phase II study of pertuzumab, trastuzumab, and weekly paclitaxel in patients with metastatic her2-overexpressing metastatic breast cancer. Cancer Res. 2012;72(24 Suppl):P5-18-20 (Abstract).
Lewis Phillips GD, Li G, Dugger DL, et al. Targeting her2-positive breast cancer with trastuzumab-dm1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90.
Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63.
Ellis P, Barrios C, Im YH, et al. MARIANNE: a phase III, randomized, multicenter study of trastuzumab emtansine (T-DM1)± pertuzumab compared with trastuzumab plus taxane for first-line treatment of human epidermal growth factor receptor 2 (HER2)-positive, progressive or recurrent locally advanced or metastatic breast cancer. J Clin Oncol. 2011;29:TPS102 (Abstract).
Urruticoechea A, Canney P, Separovic R, et al. A Phase II study of trastuzumab plus capecitabine with or without pertuzumab for HER2-positive metastatic breast cancer as second-line treatment (PHEREXA). Eur J Cancer. 2012;48:194 (Abstract).
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for her2-positive early breast cancer (neoaltto): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–40.
Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early her2-positive breast cancer (Neosphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Guarneri V, Frassoldati A, Bottini A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II cher-lob study. J Clin Oncol. 2012;30:1989–95.
Schneeweiss A, Chia S, Hickish T, et al. Neoadjuvant pertuzumab and trastuzumab concurrent or sequential with an anthracycline containing or concurrent with an anthracycline-free standard regimen: A randomized phase ii study (TRYPHAENA). Cancer Res. 2011;71(24 Suppl):S5–6 (Abstract).
Aurisicchio L, Marra E, Roscilli G, Mancini R, Ciliberto G. The promise of anti-erbb3 monoclonals as new cancer therapeutics. Oncotarget. 2012;3:744–58.
NCCN clinical practice guidelines in oncology™. Breast cancer (online). http://wwwnccnorg/professionals/physician_gls/pdf/breastpdf (2012). Accessed 15 March 2013.
Acknowledgments
No funding or sponsorship was received for the publication of this article. Prior to peer review Genentech was offered the opportunity to review this paper for scientific accuracy. Leticia De Mattos-Arruda is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
Leticia De Mattos-Arruda declares no conflicts of interest. Javier Cortes is a consultant for Roche, Eisai, Celgene, and Novartis, and has received honoraria from Roche, Eisai, Celgene, and Cephalon.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Mattos-Arruda, L., Cortes, J. Use of Pertuzumab for the Treatment of HER2-Positive Metastatic Breast Cancer. Adv Ther 30, 645–658 (2013). https://doi.org/10.1007/s12325-013-0043-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-013-0043-2