Abstract
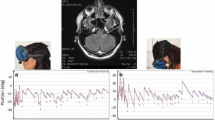
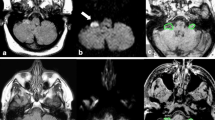
We sought to determine the cerebellar structures responsible for tilt suppression of post-rotatory nystagmus. We investigated ocular motor findings and MRI lesions in 73 patients with isolated cerebellar lesions who underwent recording of the vestibulo-ocular reflex (VOR) using rotatory chair tests. Tilt suppression of post-rotatory nystagmus was diminished in 27 patients (27/73, 37.0 %). The gains of the VOR and the TCs of per- and post-rotatory nystagmus did not differ between the patients with diminished and with normal tilt suppression. The patients with impaired tilt suppression showed perverted (“cross-coupled”) head-shaking nystagmus (pHSN) and central positional nystagmus (CPN) more frequently than those with normal responses. Tilt suppression was impaired in five (71.4 %) of the seven patients with isolated nodulus and uvular infarction. Probabilistic lesion-mapping analysis showed that the nodulus and uvula are responsible for tilt suppression. Impaired tilt suppression may be ascribed to disruption of cerebellar contribution to the vestibular velocity-storage mechanism, which integrates information from the semicircular canals and otolith organs to help derive the brain’s estimate of the head orientation relative to the pull of gravity.


Similar content being viewed by others
References
Baloh RW, Kerber K. Baloh and Honrubia’s clinical neurophysiology of the vestibular system. New York: Oxford University Press; 2010.
Cohen B, Henn V, Raphan T, Dennett D. Velocity storage, nystagmus, and visual-vestibular interactions in humans. Ann N Y Acad Sci. 1981;374(1):421–33.
Dai M, Klein A, Cohen B, Raphan T. Model-based study of the human cupular time constant. J Vestib Res. 1998;9(4):293–301.
Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006.
Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977;270:321–44.
Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011;210:407–22.
Solomon D, Cohen B. Stabilization of gaze during circular locomotion in darkness. II. Contribution of velocity storage to compensatory eye and head nystagmus in the running monkey. J Neurophysiol. 1992;67:1158–70.
Raphan T, Sturm D. Modeling the spatiotemporal organization of velocity storage in the vestibuloocular reflex by optokinetic studies. J Neurophysiol. 1991;66:1410–21.
Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228:199–202.
Raphan T, Cohen B, Henn V. Effects of gravity on rotatory nystagmus in monkeys. Ann N Y Acad Sci. 1981;374:44–55.
Kushiro K, Dai M, Kunin M, Yakushin SB, Cohen B, Raphan T. Compensatory and orienting eye movements induced by off-vertical axis rotation (OVAR) in monkeys. J Neurophysiol. 2002;88:2445–62.
Sheliga BM, Yakushin SB, Silvers A, Raphan T, Cohen B. Control of spatial orientation of the angular vestibulo-ocular reflex by the nodulus and uvula of the vestibulocerebellum. Ann N Y Acad Sci. 1999;871:94–122.
Wiest G, Deecke L, Trattnig S, Mueller C. Abolished tilt suppression of the vestibulo-ocular reflex caused by a selective uvulo-nodular lesion. Neurology. 1999;52(2):417–9.
Moon IS, Kim JS, Choi KD, Kim MJ, Oh SY, Lee H, et al. Isolated nodular infarction. Stroke. 2009;40(2):487–91.
Hain T, Zee D, Maria B. Tilt suppression of vestibulo-ocular reflex in patients with cerebellar lesions. Acta Otolaryngol. 1988;105:13–20.
Huh YE, Kim JS. Bedside evaluation of dizzy patients. J Clin Neurol. 2013;9:203–13.
Choi J-Y, Kim JH, Kim HJ, Glasauer S, Kim J-S. Central paroxysmal positional nystagmus characteristics and possible mechanisms. Neurology. 2015;84:2238–46.
Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction patterns and possible mechanisms. Neurology. 2007;68(17):1337–44.
Yang Y, Kim JS, Kim S, Kim YK, Kwak YT, Han IW. Cerebellar hypoperfusion during transient global amnesia: an MRI and oculographic study. J Clin Neurol. 2009;5(2):74–80.
Choi KD, Kim JS. Head‐shaking nystagmus in central vestibulopathies. Ann N Y Acad Sci. 2009;1164:338–43.
Jeong SH, Oh SY, Kim HJ, Koo JW, Kim JS. Vestibular dysfunction in migraine: effects of associated vertigo and motion sickness. J Neurol. 2010;257(6):905–12.
Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33(1):127–38.
Huh YE, Kim JS. Patterns of spontaneous and head-shaking nystagmus in cerebellar infarction: imaging correlations. Brain. 2011;134(12):3662–71.
Voogd J, Gerrits NM, Ruigrok TJ. Organization of the vestibulocerebellum. Ann N Y Acad Sci. 1996;781(1):553–79.
Shojaku H, Sato Y, Ikarashi K, Kawasaki T. Topographical distribution of Purkinje cells in the uvula and the nodulus projecting to the vestibular nuclei in cats. Brain Res. 1987;416(1):100–12.
Shaikh AG, Palla A, Marti S, Olasagasti I, Optican LM, Zee DS, et al. Role of cerebellum in motion perception and vestibulo-ocular reflex—similarities and disparities. Cerebellum. 2013;12(1):97–107.
Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983;278(1):29–51.
Angelaki DE, Hess BJ. Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol. 1995;73:1729–51.
Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54(6):973–85.
Meng H, Blázquez PM, Dickman JD, Angelaki DE. Diversity of vestibular nuclei neurons targeted by cerebellar nodulus inhibition. J Physiol. 2014;592:171–88.
Wearne S, Raphan T, Cohen B. Nodulo-uvular control of central vestibular dynamics determines spatial orientation of the angular vestibulo-ocular reflexa. Ann N Y Acad Sci. 1996;781:364–84.
Solomon D, Cohen B. Stimulation of the nodulus and uvula discharges velocity storage in the vestibulo-ocular reflex. Exp Brain Res. 1994;102:57–68.
Furman JM, Wall C, Pang D. Vestibular function in periodic alternating nystagmus. Brain. 1990;113(5):1425–39.
Shaikh AG. Motion perception without nystagmus—a novel manifestation of cerebellar stroke. J Stroke Cerebrovasc Dis. 2014;23:1148–56.
Shaikh AG, Marti S, Tarnutzer AA, Palla A, Crawford TO, Straumann D, et al. Ataxia telangiectasia: a “disease model” to understand the cerebellar control of vestibular reflexes. J Neurophysiol. 2011;105:3034–41.
Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999;871:205–20.
Palla A, Marti S, Straumann D. Head-shaking nystagmus depends on gravity. J Assoc Res Otolaryngol. 2005;6(1):1–8.
Waespe W, Büttner U, Henn V. Visual-vestibular interaction in the flocculus of the alert monkey. Exp Brain Res. 1981;43(3–4):337–48.
Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260(6):1576–82.
Waespe W, Cohen B, Raphan T. Role of the flocculus and paraflocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res. 1983;50:9–33.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
Author Contributions
Dr. Lee wrote the manuscript and analyzed and interpreted the data.
Drs. Choi, Park, Koo, H.J. Kim, and Zee analyzed and interpreted the data and revised the manuscript.
Dr. J.S. Kim conducted the design, and conceptualized the study, interpreted the data, and revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Study Ethics
All experiments followed the tenets of the Declaration of Helsinki and this study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1505-298-107).
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclosures
Drs. Lee, Choi, Park, and Koo and H.J. Kim report no disclosure.
Dr. J.S. Kim serves as an associate editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, Journal of Neurology, and Medicine and received research support from SK Chemicals, Co. Ltd.
Dr. Zee receives research support from the National Institutes of Health and is an Associate Editor of Frontiers in Neuro-otology and a member of the Editorial Board of The Cerebellum. He received speaker’s honoraria from Abbott, Micromed, Sun pharmaceuticals, and the American Academy of Neurology.
Rights and permissions
About this article
Cite this article
Lee, SU., Choi, JY., Kim, HJ. et al. Impaired Tilt Suppression of Post-Rotatory Nystagmus and Cross-Coupled Head-Shaking Nystagmus in Cerebellar Lesions: Image Mapping Study. Cerebellum 16, 95–102 (2017). https://doi.org/10.1007/s12311-016-0772-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0772-2