Abstract
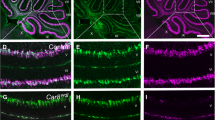
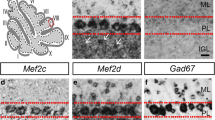
Recent studies have found that in the cerebellum, the δ2 glutamate receptor (GluRδ2) plays a key role in regulating the differentiation of parallel fiber-Purkinje synapses and mediating key physiological functions in the granule cell-Purkinje cell circuit. In the hotfoot mutant or GluRδ2 knockout mice, the absence of GluRδ2 expression results in impaired motor-related tasks, ataxia, and disruption of long-term depression at parallel fiber-Purkinje cell synapses. The goal of this study was to determine the long-term consequences of deletion of GluRδ2 expression in the hotfoot mutant (GluRδ2ho/ho) on Purkinje and granule cell survival and Purkinje cell dendritic differentiation. Quantitative estimates of Purkinje and granule cell numbers in 3-, 12-, and 20-month-old hotfoot mutants and wild-type controls showed that Purkinje cell numbers are within control values at 3 and 12 months in the hotfoot mutant but reduced by 20 % at 20 months compared with controls. In contrast, the number of granule cells is significantly reduced from 3 months onwards in GluRδ2ho/ho mutant mice compared to wild-type controls. Although the overall structure of Purkinje cell dendrites does not appear to be altered, there is a significant 27 % reduction in the cross-sectional area of Purkinje cell dendritic trees in the 20-month-old GluRδ2ho/ho mutants. The interpretation of the results is that the GluRδ2 receptor plays an important role in the long-term organization of the granule-Purkinje cell circuit through its involvement in the regulation of parallel fiber-Purkinje cell synaptogenesis and in the normal functioning of this critical cerebellar circuit.







Similar content being viewed by others
References
Yuzaki M. The delta2 glutamate receptor: 10 years later. Neurosci Res. 2003;46(1):11–22.
Vogel MW, Caston J, Yuzaki M, Mariani J. The Lurcher mouse: fresh insights from an old mutant. Brain Res. 2007;1140:4–18.
Yuzaki M. The delta2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cells. Cerebellum. 2004;3(2):89–93.
Lomeli H, Sprengel R, Lauris DJ, Kohr G, Herb A, Seeburg PH, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–22.
Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel d2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Comm. 1993;197:1267–76.
Kohda K, Kakegawa W, Yuzaki M. Unlocking the secrets of the d2 glutamate receptor. Commun Integr Biol. 2013;66:e26466.
Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant mice. Cell. 1995;81:245–52.
Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, et al. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21(24):9701–12.
Kato AS, Knierman MD, Siuda ER, Isaac JT, Nisenbaum ES, Bredt DS. Glutamate receptor delta2 associates with metabotropic glutamate receptor 1 (mGluR1), protein kinase Cgamma, and canonical transient receptor potential 3 and regulates mGluR1-mediated synaptic transmission in cerebellar Purkinje neurons. J Neurosci. 2012;32(44):15296–308.
Kakegawa W, Kohda K, Yuzaki M. The {delta}2 “ionotropic” glutamate receptor functions as a non-ionotropic receptor to control cerebellar synaptic plasticity. J Physiol. 2007;16:16.
Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, et al. D-serine regulates cerebellar LTD and motor coordination through the delta2 glutamate receptor. Nat Neurosci. 2011;14(5):603–11.
Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, et al. Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc Natl Acad Sci U S A. 2007;104(35):14116–21.
Ady V, Perroy J, Tricoire L, Piochon C, Dadak S, Chen X, et al. Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors. EMBO Rep. 2014;15(1):103–9.
Ito-Ishida A, Miyazaki T, Miura E, Matsuda K, Watanabe M, Yuzaki M, et al. Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron. 2012;76(3):549–64.
Matsuda K, Yuzaki M. Cbln1 and the delta2 glutamate receptor—an orphan ligand and an orphan receptor find their partners. Cerebellum. 2012;11(1):78–84.
Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32(2):191–7.
Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, et al. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328(5976):363–8.
Matsuda K, Yuzaki M. Cbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regions. Eur J Neurosci. 2011;33(8):1447–61.
Yuzaki M. Cbln1 and its family proteins in synapse formation and maintenance. Curr Opin Neurobiol. 2011;21(2):215–20.
Kohda K, Kakegawa W, Matsuda S, Yamamoto T, Hirano H, Yuzaki M. The delta2 glutamate receptor gates long-term depression by coordinating interactions between two AMPA receptor phosphorylation sites. Proc Natl Acad Sci U S A. 2013;110(10):E948–57.
Guastavino JM, Sotelo C, Damez-Kinselle I. Hot-foot murine mutation: behavioral effects and neuroanatomical alterations. Brain Res. 1990;523(2):199–210.
Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurobiological effects of a null mutation depend on genetic context: comparison between two hotfoot alleles of the delta-2 ionotropic glutamate receptor. Neuroscience. 2001;105(2):443–55.
Utine GE, Haliloglu G, Salanci B, Cetinkaya A, Kiper PO, Alanay Y, et al. A homozygous deletion in GRID2 causes a human phenotype with cerebellar ataxia and atrophy. J Child Neurol. 2013;28(7):926–32.
Hills LB, Masri A, Konno K, Kakegawa W, Lam AT, Lim-Melia E, et al. Deletions in GRID2 lead to a recessive syndrome of cerebellar ataxia and tonic upgaze in humans. Neurology. 2013;81(16):1378–86.
Maier A, Klopocki EPDRN, Horn DPDM, Tzschach APDM, Holm T, Meyer R, et al. De novo partial deletion in GRID2 presenting with complicated spastic paraplegia. Muscle Nerve. 2014;49(2):289–92.
Coutelier M, Burglen L, Mundwiller E, Abada-Bendib M, Rodriguez D, Chantot-Bastaraud S, et al. GRID2 mutations span from congenital to mild adult-onset cerebellar ataxia. Neurology. 2015;84(17):1751–9.
Lalonde R, Hayzoun K, Selimi F, Mariani J, Strazielle C. Motor coordination in mice with hotfoot, Lurcher, and double mutations of the Grid2 gene encoding the delta-2 excitatory amino acid receptor. Physiol Behav. 2003;80(2–3):333–9.
Selimi F, Lohof AM, Heitz S, Lalouette A, Jarvis CI, Bailly Y, et al. Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells. Neuron. 2003;37(5):813–9.
McFarland R, Blokhin A, Sydnor J, Mariani J, Vogel MW. Oxidative stress, nitric oxide, and the mechanisms of cell death in Lurcher Purkinje cells. Dev Neurobiol. 2007;67:1032–46.
McFarland R, Zanjani HS, Mariani J, Vogel MW. Changes in the distribution of the a3 Na+/K+ ATPase subunit in heterozygous Lurcher Purkinje cells as a genetic model of chronic depolarization during development. Int J Cell Biol. 2014
Zanjani HS, Vogel MW, Delhaye-Bouchaud N, Martinou JC, Mariani J. Increased cerebellar Purkinje cell numbers in mice overexpressing a human bcl-2 transgene. J Comp Neurol. 1996;374:332–41.
Wetts R, Herrup K. Cerebellar Purkinje cells are descended from a small number of progenitors committed during early development: quantitative analysis of lurcher chimeric mice. J Neurosci. 1982;2:1494–8.
Herrup K, Sunter K. Cell lineage dependent and independent control of Purkinje cell number in the mammalian CNS: further quantitative studies of lurcher chimeric mice. Dev Biol. 1986;117:417–27.
Hendry IA. A method to correct adequately for the change in neuronal size when estimating neuronal numbers following nerve growth factor treatment. J Neurocytol. 1976;5:337–49.
Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimeric mice. II. Granule cell death. Brain Res. 1982;250:358–63.
Vogel MW, Herrup K. Numerical matching in the mammalian CNS: lack of a competitive advantage of early over late-generated cerebellar granule cells. J Comp Neurol. 1989;283:118–28.
Zanjani HS, Vogel MW, Delhaye-Bouchaud N, Martinou JC, Mariani J. Increased inferior olivary neuron and cerebellar granule cell numbers in transgenic mice overexpressing the human Bcl-2 gene. J Neurobiol. 1997;32(5):502–16.
Fan H, Favero M, Vogel MW. Elimination of Bax expression in mice increases cerebellar Purkinje cell numbers but not the number of granule cells. J Comp Neurol. 2001;436:82–91.
Zanjani H, Lemaigre-Dubreuil Y, Tillakaratne NJ, Blokhin A, McMahon RP, Tobin AJ, et al. Cerebellar Purkinje cell loss in aging Hu-Bcl-2 transgenic mice. J Comp Neurol. 2004;475(4):481–92.
Popken GJ, Farel FB. Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386:8–15.
Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7.
Farel PB. Trust, but verify: the necessity of empirical verification in quantitative neurobiology. Anat Rec. 2002;269(3):157–61.
von Bartheld CS. Counting particles in tissue sections: choices of methods and importance of calibration to minimize biases. Histol Histopathol. 2002;17:639–48.
Caddy KWT, Herrup K. Studies of the dendritic tree of wild-type cerebellar Purkinje cells in lurcher chimeric mice. J Comp Neurol. 1990;297:121–31.
Yuzaki M. New (but old) molecules regulating synapse integrity and plasticity: Cbln1 and the delta2 glutamate receptor. Neuroscience. 2009;162(3):633–43.
Yuzaki M. The ins and outs of GluD2—why and how Purkinje cells use the special glutamate receptor. Cerebellum. 2011;11(2):438–9.
Mandolesi G, Autuori E, Cesa R, Premoselli F, Cesare P, Strata P. GluRdelta2 expression in the mature cerebellum of hotfoot mice promotes parallel fiber synaptogenesis and axonal competition. PLoS One. 2009;4(4):e5243.
Mandolesi G, Cesa R, Autuori E, Strata P. An orphan ionotropic glutamate receptor: the delta2 subunit. Neuroscience. 2009;158(1):67–77.
Mishina M, Uemura T, Yasumura M, Yoshida T. Molecular mechanism of parallel fiber-Purkinje cell synapse formation. Front Neural Circuits. 2012;6:90.
Sarna JR, Hawkes R. Patterned Purkinje cell death in the cerebellum. Prog Neurobiol. 2003;70(6):473–507.
Sotelo C. Cerebellar synaptogenesis: what we can learn from mutant mice. J Exp Biol. 1990;153:225–49.
Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Neurodegeneration in Lurcher mice occurs via multiple cell death pathways. J Neurosci. 2000;20(10):3687–94.
Herrup K, Shojaeian-Zanjani H, Panzini L, Sunter K, Mariani J. The numerical matching of source and target populations in the CNS: the inferior olive to Purkinje cell projection. Dev Brain Res. 1996;96:28–35.
Dahmane N, Ruiz-i-Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126(14):3089–100.
Herrup K. Role of staggerer gene in determining cell number in cerebellar cortex. I. Granule cell death is an indirect consequence of staggerer gene action. Brain Res. 1983;11:267–74.
Herrup K, Mullen RJ. Staggerer chimeras: intrinsic nature of Purkinje cell defects and implications for normal cerebellar development. Brain Res. 1979;178:443–57.
Herrup K, Sunter K. Numerical matching during cerebellar development: quantitative analysis of granule cell death in staggerer mouse chimeras. J Neurosci. 1987;7:829–36.
Smeyne RJ, Chu T, Lewin A, Bian F, Crisman SS, Kunsch C, et al. Local control of granule cell generation by cerebellar Purkinje cells. Mol Cell Neurosci. 1995;6:230–51.
Sonmez E, Herrup K. Role of staggerer gene in determining cell number in cerebellar cortex. II. Granule cell death and persistence of the external granule cell layer in young mouse chimeras. Brain Res. 1984;12:271–83.
Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9(8):445–8.
Vogel MW, Sunter K, Herrup K. Numerical matching between granule and Purkinje cells in lurcher chimeric mice: a hypothesis for the trophic rescue of granule cells from target related cell death. J Neurosci. 1989;9:3454–62.
Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic hedgehog. Neuron. 1999;22(1):103–14.
Wetts R, Herrup K. Direct correlation between Purkinje and granule cell number in the cerebella of lurcher chimeras and wild-type mice. Dev Brain Res. 1983;10:41–7.
Selimi F, Doughty M, Delhaye-Bouchaud N, Mariani J. Target-related and intrinsic neuronal death in Lurcher mutant mice are both mediated by caspase-3 activation. J Neurosci. 2000;20:992–1000.
Rios I, Alvarez-Rodriguez R, Marti E, Pons S. Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development. 2004;131(13):3159–68.
Doughty ML, Lohof A, Campana A, Delhaye-Bouchaud N, Mariani J. Neurotrophin-3 promotes cerebellar granule cell exit from the EGL. Eur J Neurosci. 1998;10(9):3007–11.
Williams R, Herrup K. The control of neuron number. Annu Rev Neurosci. 1988;11:423–53.
Bradley PM, Berry M. The Purkinje cell dendritic tree in mutant mouse cerebellum. A quantitative Golgi study of weaver and staggerer mice. Brain Res. 1978;142:135–41.
Hirano A, Dembitzer HB. The fine structure of staggerer cerebellum. J Neuropathol Exp Neurol. 1975;34:1–11.
Landis DMD, Sidman RL. Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice. J Comp Neurol. 1978;179:831–64.
Sotelo C, Changeux J-P. Transsynaptic degeneration “en cascade” in the cerebellar cortex of staggerer mutant mice. Brain Res. 1974;67:519–26.
Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287(1020):167–201.
Swisher DA, Wilson DB. Cerebellar histogenesis in the Lurcher (Lc) mutant mouse. J Comp Neurol. 1977;173:205–17.
Landis SC, Mullen RJ. The development and degeneration of Purkinje cells in pcd mutant mice. J Comp Neurol. 1978;177(1):125–43.
Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976;73:208–12.
Triarhou LC. Biological clues on neuronal degeneration based on theoretical fits of decay patterns: towards a mathematical neuropathology. Folia Neuropathol. 2010;48(1):3–10.
Triarhou LC. Rate of neuronal fallout in a transsynaptic cerebellar model. Brain Res Bull. 1998;47(3):219–22.
Kurihara H, Hashimoto K, Kano M, Takayama C, Sakimura K, Mishina M, et al. Impaired parallel fiber→Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor delta2 subunit. J Neurosci. 1997;17:9613–23.
Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, et al. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J Neurosci. 2002;22(19):8487–503.
Yuzaki M. Cerebellar LTD vs. motor learning-Lessons learned from studying GluD2. Neural Netw. 2012;47(2012):36–41.
Kaneko M, Yamaguchi K, Eiraku M, Sato M, Takata N, Kiyohara Y, et al. Remodeling of monoplanar Purkinje cell dendrites during cerebellar circuit formation. PLoS One. 2011;6(5):e20108.
Metzger F. Molecular and cellular control of dendrite maturation during brain development. Curr Mol Pharmacol. 2009;3(1):1–11.
Tanaka M. Dendrite formation of cerebellar Purkinje cells. Neurochem Res. 2009;34(12):2078–88.
Boukhtouche F, Janmaat S, Vodjdani G, Gautheron V, Mallet J, Dusart I, et al. Retinoid-related orphan receptor alpha controls the early steps of Purkinje cell dendritic differentiation. J Neurosci. 2006;26:1531–8.
Boukhtouche F, Brugg B, Wehrlé R, Bois-Joyeux B, Danan JL, Dusart I, et al. Induction of early Purkinje cell dendritic differentiation by thyroid hormone requires RORα. Neural Dev. 2010;5:18.
Takeo YH, Kakegawa W, Miura E, Yuzaki M. RORa regulates multiple aspects of dendrite development in cerebellar Purkinje cells in vivo. J Neurosci. 2015;35:12518–34.
Acknowledgments
This work was supported by a UPMC LIA (Laboratoire international associé) grant to JM and by a visiting professor grant of UPMC to MWV. We thank Corinne Nantet for her histological assistance. This article was prepared while MWV was employed at the University of Maryland School of Medicine. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Authors’ Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Hadi Zanjani, Michael W. Vogel, and Jean Mariani were responsible for the study concept and design; Hadi Zanjani for the acquisition of data; Hadi Zanjani, Michael W. Vogel, and Jean Mariani for the analysis and interpretation of data; Hadi Zanjani and Michael W. Vogel for the drafting of the manuscript; Hadi Zanjani, Michael W. Vogel, and Jean Mariani for the critical revision of the manuscript for intellectual content; Hadi Zanjani and Michael W. Vogel for the statistical analysis; Jean Mariani for the obtained funding; Hadi Zanjani and Jean Mariani for the administrative, technical, and material support; and Hadi Zanjani, Michael W. Vogel, and Jean Mariani for the study supervision.
Rights and permissions
About this article
Cite this article
Zanjani, H.S., Vogel, M.W. & Mariani, J. Deletion of the GluRδ2 Receptor in the Hotfoot Mouse Mutant Causes Granule Cell Loss, Delayed Purkinje Cell Death, and Reductions in Purkinje Cell Dendritic Tree Area. Cerebellum 15, 755–766 (2016). https://doi.org/10.1007/s12311-015-0748-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0748-7