Abstract
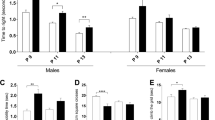
There have been suggestions that maternal immune activation is associated with alterations in motor behavior in offspring. To explore this further, we treated pregnant mice with polyinosinic:polycytidylic acid (poly(I:C)), a viral mimetic that activates the innate immune system, or saline on embryonic days 13–15. At postnatal day (P) 18, offspring cerebella were collected from perfused brains and immunostained as whole-mounts for zebrin II. Measurements of zebrin II+/− stripes in both sexes indicated that prenatal poly(I:C)-exposed offspring had significantly wider stripes; this difference was also seen in similarly treated offspring in adulthood (~P120). When sagittal sections of the cerebellum were immunostained for calbindin and Purkinje cell numbers were counted, we observed greater numbers of Purkinje cells in poly(I:C) offspring at both P18 and ~ P120. In adolescence (~P40), both male and female prenatal poly(I:C)-exposed offspring exhibited poorer performance on the rotarod and ladder rung tests; deficits in performance on the latter test persisted into adulthood. Offspring of both sexes from poly(I:C) dams also exhibited impaired social interaction in adolescence, but this difference was no longer apparent in adulthood. Our results suggest that maternal immune exposure at a critical time of cerebellum development can enhance neuronal survival or impair normal programmed cell death of Purkinje cells, with lasting consequences on cerebellar morphology and a variety of motor and non-motor behaviors.







Similar content being viewed by others
References
Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–80. doi:10.1001/archpsyc.61.8.774.
Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–92.
Susser ES, Schaefer CA, Brown AS, Begg MD, Wyatt RJ. The design of the prenatal determinants of schizophrenia study. Schizophr Bull. 2000;26:257–73.
Miller MT, Stromland K, Ventura L, Johansson M, Bandim JM, Gillberg C. Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int J Dev Neurosci. 2005;23:201–19. doi:10.1016/j.ijdevneu.2004.06.007.
Rodier PM, Hyman SL. Early environmental factors in autism. Ment Retard Dev Disabil Res Rev. 1998;4:121–8.
Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33. doi:10.1203/PDR.0b013e318212c196.
Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–21. doi:10.1096/fj.04-3569fje.
Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006;31:1910–8. doi:10.1038/sj.npp.1301004.
Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–74. doi:10.1002/glia.20117.
Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–12. doi:10.1016/j.jneuroim.2004.10.008.
Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–15. doi:10.1016/j.bbi.2010.12.017.
Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72.
Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802.
Arrode-Bruses G, Bruses JL. Maternal immune activation by poly I:C induces expression of cytokines IL-1beta and IL-13, chemokine MCP-1 and colony stimulating factor VEGF in fetal mouse brain. J Neuroinflammation. 2012;9:83. doi:10.1186/1742-2094-9-83.
Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–88. doi:10.1016/j.bbi.2005.11.003.
Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–86. doi:10.1016/j.bbi.2007.09.012.
Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33.
Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008;33:441–56. doi:10.1038/sj.npp.1301413.
Sharma A, Valadi N, Miller AH, Pearce BD. Neonatal viral infection decreases neuronal progenitors and impairs adult neurogenesis in the hippocampus. Neurobiol Dis. 2002;11:246–56.
Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, et al. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4, e6354. doi:10.1371/journal.pone.0006354.
Smith SE, Elliott RM, Anderson MP. Maternal immune activation increases neonatal mouse cortex thickness and cell density. J Neuroimmune Pharm. 2012;7:529–32. doi:10.1007/s11481-012-9372-1.
Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:2462–78. doi:10.1038/npp.2010.129.
Wang H, Meng XH, Ning H, Zhao XF, Wang Q, Liu P, et al. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2010;192:245–51. doi:10.1016/j.toxlet.2009.10.030.
Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6.
Limperopoulos C, du Plessis AJ. Disorders of cerebellar growth and development. Curr Opin Pediatr. 2006;18:621–7. doi:10.1097/MOP.0b013e32801080e8.
Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. 2009;24:1085–104. doi:10.1177/0883073809338067.
Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J Comp Neurol. 1990;291:538–52. doi:10.1002/cne.902910405.
Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10:422–34. doi:10.1007/s12311-010-0208-3.
Sillitoe RV, Marzban H, Larouche M, Zahedi S, Affanni J, Hawkes R. Conservation of the architecture of the anterior lobe vermis of the cerebellum across mammalian species. Prog Brain Res. 2005;148:283–97. doi:10.1016/S0079-6123(04)48022-4.
Armstrong C, Hawkes R. Pattern formation in the cerebellum. Morgan & Claypool Life Sciences; 2013.
Armstrong CL, Hawkes R. Pattern formation in the cerebellar cortex. Biochem Cell Biol. 2000;78:551–62.
Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, et al. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–29. doi:10.1242/dev.02437.
Leclerc N, Gravel C, Hawkes R. Development of parasagittal zonation in the rat cerebellar cortex: MabQ113 antigenic bands are created postnatally by the suppression of antigen expression in a subset of Purkinje cells. J Comp Neurol. 1988;273:399–420. doi:10.1002/cne.902730310.
Hawkes R, Mascher C. The development of molecular compartmentation in the cerebellar cortex. Acta Anat (Basel). 1994;151:139–49.
Seil FJ, Johnson ML, Hawkes R. Molecular compartmentation expressed in cerebellar cultures in the absence of neuronal activity and neuron-glia interactions. J Comp Neurol. 1995;356:398–407. doi:10.1002/cne.903560307.
Wassef M, Sotelo C, Thomasset M, Granholm AC, Leclerc N, Rafrafi J, et al. Expression of compartmentation antigen zebrin I in cerebellar transplants. J Comp Neurol. 1990;294:223–34. doi:10.1002/cne.902940207.
Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409.
Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–42.
Hashimoto M, Mikoshiba K. Mediolateral compartmentalization of the cerebellum is determined on the “birth date” of Purkinje cells. J Neurosci. 2003;23:11342–51.
Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–96.
Namba K, Sugihara I, Hashimoto M. Close correlation between the birth date of Purkinje cells and the longitudinal compartmentalization of the mouse adult cerebellum. J Comp Neurol. 2011;519:2594–614. doi:10.1002/cne.22640.
Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi:10.1146/annurev.neuro.20.1.61.
Larouche M, Che PM, Hawkes R. Neurogranin expression identifies a novel array of Purkinje cell parasagittal stripes during mouse cerebellar development. J Comp Neurol. 2006;494:215–27. doi:10.1002/cne.20791.
Oberdick J, Baader SL, Schilling K. From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. Trends Neurosci. 1998;21:383–90.
Sillitoe RV, Joyner AL. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol. 2007;23:549–77. doi:10.1146/annurev.cellbio.23.090506.123237.
Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev Neurobiol. 2012;72:1335–48. doi:10.1002/dneu.22043.
Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–23. doi:10.1016/j.bbi.2008.07.012.
Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–34.
Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–25. doi:10.1016/j.neubiorev.2004.10.006.
Nguon K, Ladd B, Baxter MG, Sajdel-Sulkowska EM. Sexual dimorphism in cerebellar structure, function, and response to environmental perturbations. Prog Brain Res. 2005;148:341–51. doi:10.1016/S0079-6123(04)48027-3.
Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. J Histochem Cytochem. 2002;50:235–44.
White JJ, Reeber SL, Hawkes R and Sillitoe RV. Wholemount immunohistochemistry for revealing complex brain topography. J Vis Exp. 2012;62:e4042. doi 10.3791/4042
Celio MR. Calbindin D-28 k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475.
Tano D, Napieralski JA, Eisenman LM, Messer A, Plummer J, Hawkes R. Novel developmental boundary in the cerebellum revealed by zebrin expression in the lurcher (Lc/+) mutant mouse. J Comp Neurol. 1992;323:128–36. doi:10.1002/cne.903230111.
Marzban H, Zahedi S, Sanchez M, Hawkes R. Antigenic compartmentation of the cerebellar cortex in the syrian hamster Mesocricetus auratus. Brain Res. 2003;974:176–83.
Caston J, Jones N, Stelz T. Role of preoperative and postoperative sensorimotor training on restoration of the equilibrium behavior in adult mice following cerebellectomy. Neurobiol Learn Mem. 1995;64:195–202. doi:10.1006/nlme.1995.0002.
Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–6.
Stroobants S, Gantois I, Pooters T, D’Hooge R. Increased gait variability in mice with small cerebellar cortex lesions and normal rotarod performance. Behav Brain Res. 2013;241:32–7. doi:10.1016/j.bbr.2012.11.034.
Hunsaker MR, von Leden RE, Ta BT, Goodrich-Hunsaker NJ, Arque G, Kim K, et al. Motor deficits on a ladder rung task in male and female adolescent and adult CGG knock-in mice. Behav Brain Res. 2011;222:117–21. doi:10.1016/j.bbr.2011.03.039.
Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–79.
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16. doi:10.1016/j.bbi.2012.01.011.
Xuan IC, Hampson DR. Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS One. 2014;9, e104433. doi:10.1371/journal.pone.0104433.
Wallace K, Veerisetty S, Paul I, May W, Miguel-Hidalgo JJ, Bennett W. Prenatal infection decreases calbindin, decreases Purkinje cell volume and density and produces long-term motor deficits in Sprague-Dawley rats. Dev Neurosci. 2010;32:302–12. doi:10.1159/000319506.
Goffinet AM. The embryonic development of the cerebellum in normal and reeler mutant mice. Anat Embryol (Berl). 1983;168:73–86.
Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701.
Gallagher E, Howell BW, Soriano P, Cooper JA, Hawkes R. Cerebellar abnormalities in the disabled (mdab1-1) mouse. J Comp Neurol. 1998;402:238–51.
Goldowitz D, Cushing RC, Laywell E, D’Arcangelo G, Sheldon M, Sweet HO, et al. Cerebellar disorganization characteristic of reeler in scrambler mutant mice despite presence of reelin. J Neurosci. 1997;17:8767–77.
Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–7. doi:10.1038/39607.
Chung SH, Marzban H, Croci L, Consalez GG, Hawkes R. Purkinje cell subtype specification in the cerebellar cortex: early B-cell factor 2 acts to repress the zebrin II-positive Purkinje cell phenotype. Neuroscience. 2008;153:721–32. doi:10.1016/j.neuroscience.2008.01.090.
Croci C, Fasano S, Superchi D, Perani L, Martellosio A, Brambilla R, et al. Cerebellar neurons and glial cells are transducible by lentiviral vectors without decrease of cerebellar functions. Dev Neurosci. 2006;28:216–21. doi:10.1159/000091919.
Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compartmentation. J Comp Neurol. 2001;429:7–21.
Khan D, Fernando P, Cicvaric A, Berger A, Pollak A, Monje FJ, et al. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl Psychiatry. 2014;4, e363. doi:10.1038/tp.2013.132.
Liu YH, Lai WS, Tsay HJ, Wang TW, Yu JY. Effects of maternal immune activation on adult neurogenesis in the subventricular zone-olfactory bulb pathway and olfactory discrimination. Schizophr Res. 2013;151:1–11. doi:10.1016/j.schres.2013.09.007.
De Miranda J, Yaddanapudi K, Hornig M, Villar G, Serge R and Lipkin WI. Induction of Toll-like receptor 3-mediated immunity during gestation inhibits cortical neurogenesis and causes behavioral disturbances. MBio 2010;1:200176–10 doi: 10.1128/mBio.00176-10.
Dusart I, Flamant F. Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Front Neuroanat. 2012;6:11. doi:10.3389/fnana.2012.00011.
Zanjani HS, Vogel MW, Delhaye-Bouchaud N, Martinou JC, Mariani J. Increased cerebellar Purkinje cell numbers in mice overexpressing a human bcl-2 transgene. J Comp Neurol. 1996;374:332–41. doi:10.1002/(SICI)1096-9861(19961021)374:3<332::AID-CNE2>3.0.CO;2-2.
Vogel MW. Cell death, Bcl-2, Bax, and the cerebellum. Cerebellum. 2002;1:277–87. doi:10.1080/147342202320883588.
Fan H, Favero M, Vogel MW. Elimination of Bax expression in mice increases cerebellar purkinje cell numbers but not the number of granule cells. J Comp Neurol. 2001;436:82–91.
Goswami J, Martin LA, Goldowitz D, Beitz AJ, Feddersen RM. Enhanced Purkinje cell survival but compromised cerebellar function in targeted anti-apoptotic protein transgenic mice. Mol Cell Neurosci. 2005;29:202–21. doi:10.1016/j.mcn.2005.02.010.
Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–76.
Ashwell K. Microglia and cell death in the developing mouse cerebellum. Brain Res Dev Brain Res. 1990;55:219–30.
Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–25. doi:10.1002/glia.20616.
Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi:10.1002/glia.20810.
Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27:22–32. doi:10.1016/j.bbi.2012.09.003.
Torres-Aleman I, Pons S, Santos-Benito FF. Survival of Purkinje cells in cerebellar cultures is increased by insulin-like growth factor I. Eur J Neurosci. 1992;4:864–9.
Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J Neurosci. 1991;11:462–71.
Mount HT, Elkabes S, Dreyfus CF, Black IB. Differential involvement of metabotropic and p75 neurotrophin receptors in effects of nerve growth factor and neurotrophin-3 on cultured Purkinje cell survival. J Neurochem. 1998;70:1045–53.
Baker NL, Carlo Russo V, Bernard O, D’Ercole AJ, Werther GA. Interactions between bcl-2 and the IGF system control apoptosis in the developing mouse brain. Brain Res Dev Brain Res. 1999;118:109–18.
Croci L, Barili V, Chia D, Massimino L, van Vugt R, Masserdotti G, et al. Local insulin-like growth factor I expression is essential for Purkinje neuron survival at birth. Cell Death Differ. 2011;18:48–59. doi:10.1038/cdd.2010.78.
Tolbert DL, Clark BR. GDNF and IGF-I trophic factors delay hereditary Purkinje cell degeneration and the progression of gait ataxia. Exp Neurol. 2003;183:205–19.
Heaton MB, Mitchell JJ, Paiva M. Overexpression of NGF ameliorates ethanol neurotoxicity in the developing cerebellum. J Neurobiol. 2000;45:95–104.
Neveu I, Arenas E. Neurotrophins promote the survival and development of neurons in the cerebellum of hypothyroid rats in vivo. J Cell Biol. 1996;133:631–46.
Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi:10.1038/sj.mp.4001748.
Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36.
Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi:10.1523/JNEUROSCI. 2178-07.2007.
Girard S, Tremblay L, Lepage M, Sebire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol. 2010;184:3997–4005. doi:10.4049/jimmunol.0903349.
Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701. doi:10.1113/jphysiol.2005.102939.
Kirsten TB, Lippi LL, Bevilacqua E, Bernardi MM. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1Beta levels in adult rat offspring: relevance to autism. PLoS One. 2013;8:e82244. doi:10.1371/journal.pone.0082244.
Huang CC, Shih MC, Hsu NC, Chien Y, Chung BC. Fetal glucocorticoid synthesis is required for development of fetal adrenal medulla and hypothalamus feedback suppression. Endocrinology. 2012;153:4749–56. doi:10.1210/en.2012-1258.
Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi:10.1111/j.1600-0897.2008.00682.x.
Howland JG, Cazakoff BN, Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–98. doi:10.1016/j.neuroscience.2011.11.011.
Lante F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira MC, et al. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med. 2007;42:1231–45. doi:10.1016/j.freeradbiomed.2007.01.027.
Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372–83. doi:10.1038/mp.2008.44.
Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi:10.1038/tp.2013.16.
Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302.
Airey DC, Lu L, Williams RW. Genetic control of the mouse cerebellum: identification of quantitative trait loci modulating size and architecture. J Neurosci. 2001;21:5099–109.
Herrup K. Cell lineage relationships in the development of the mammalian CNS: role of cell lineage in control of cerebellar Purkinje cell number. Dev Biol. 1986;115:148–54.
Wahlsten D, Andison M. Patterns of cerebellar foliation in recombinant inbred mice. Brain Res. 1991;557:184–9.
Vogel MW, Sunter K, Herrup K. Numerical matching between granule and Purkinje cells in lurcher chimeric mice: a hypothesis for the trophic rescue of granule cells from target-related cell death. J Neurosci. 1989;9:3454–62.
Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34:1697–710. doi:10.1111/j.1460-9568.2011.07894.x.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi:10.1146/annurev.neuro.31.060407.125606.
Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–54.
Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–28. doi:10.1093/cercor/bhp091.
Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410. doi:10.1007/s12311-010-0204-7.
Wang SS, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–32. doi:10.1016/j.neuron.2014.07.016.
Patterson PH. Modeling autistic features in animals. Pediatr Res. 2011;69:34R–40. doi:10.1203/PDR.0b013e318212b80f.
Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–7. doi:10.1016/j.neuro.2007.01.014.
Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–30. doi:10.1007/s10803-010-1006-y.
Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010;40:1227–40. doi:10.1007/s10803-010-0981-3.
Green D, Charman T, Pickles A, Chandler S, Loucas T, Simonoff E, et al. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol. 2009;51:311–6. doi:10.1111/j.1469-8749.2008.03242.x.
Hilton CL, Zhang Y, Whilte MR, Klohr CL, Constantino J. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism. 2012;16:430–41. doi:10.1177/1362361311423018.
Becker EB, Stoodley CJ. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34. doi:10.1016/B978-0-12-418700-9.00001-0.
Sacrey LA, Germani T, Bryson SE, Zwaigenbaum L. Reaching and grasping in autism spectrum disorder: a review of recent literature. Front Neurol. 2014;5:6. doi:10.3389/fneur.2014.00006.
Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi:10.1016/j.tins.2007.12.005.
Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–7. doi:10.1016/j.ijdevneu.2004.09.006.
Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, et al. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–8. doi:10.1007/s10803-005-5037-8.
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54.
Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, et al. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25:1–18.
Limperopoulos C, Bassan H, Gauvreau K, Robertson RLJ, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–93.
Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51.
Wegiel J, Kuchna I, Nowicki K, Imaki H, Marchi E, Ma SY, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119:755–70. doi:10.1007/s00401-010-0655-4.
Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–67. doi:10.1080/14734220701490995.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51. doi:10.1038/nature11310.
Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14:221–8. doi:10.1002/ddrr.25.
Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron. 2005;47:339–52. doi:10.1016/j.neuron.2005.07.005.
Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–32. doi:10.1126/science.1224159.
Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat Commun. 2014;5:5586. doi:10.1038/ncomms6586.
Craig MC, Zaman SH, Daly EM, Cutter WJ, Robertson DM, Hallahan B, et al. Women with autistic-spectrum disorder: magnetic resonance imaging study of brain anatomy. Br J Psychiatry. 2007;191:224–8. doi:10.1192/bjp.bp.106.034603.
Noriuchi M, Kikuchi Y, Yoshiura T, Kira R, Shigeto H, Hara T, et al. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010;1362:141–9. doi:10.1016/j.brainres.2010.09.051.
Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–16. doi:10.1007/s12311-008-0043-y.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, et al. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi:10.1007/s12311-012-0355-9.
Acknowledgments
This work was supported by Canadian Institutes of Health Research grants to RH and QJP. SAR and QJP were supported by personnel awards from Alberta Innovates - Health Solutions. We thank Dr. Mio Tsutsui for valuable technical support and the RUN facility of the Hotchkiss Brain Institute for access to behavioral facilities.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest either directly or indirectly related to this research. This research was funded by the Canadian Institutes of Health Research (MOP 191318).
Research Involving Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Calgary where the research was performed and were approved by the University of Calgary Animal Care Committee in accordance with guidelines from the Canadian Council on Animal Care.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tooka Aavani and Shadna A. Rana are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Table 1
(DOCX 148 kb)
Rights and permissions
About this article
Cite this article
Aavani, T., Rana, S.A., Hawkes, R. et al. Maternal Immune Activation Produces Cerebellar Hyperplasia and Alterations in Motor and Social Behaviors in Male and Female Mice. Cerebellum 14, 491–505 (2015). https://doi.org/10.1007/s12311-015-0669-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0669-5