Abstract
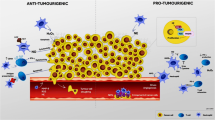
Neutrophils are myeloid cells that constitute 50–70 % of all white blood cells in the human circulation. Traditionally, neutrophils are viewed as the first line of defense against infections and as a major component of the inflammatory process. In addition, accumulating evidence suggest that neutrophils may also play a key role in multiple aspects of cancer biology. The possible involvement of neutrophils in cancer prevention and promotion was already suggested more than half a century ago, however, despite being the major component of the immune system, their contribution has often been overshadowed by other immune components such as lymphocytes and macrophages. Neutrophils seem to have conflicting functions in cancer and can be classified into anti-tumor (N1) and pro-tumor (N2) sub-populations. The aim of this review is to discuss the varying nature of neutrophil function in the cancer microenvironment with a specific emphasis on the mechanisms that regulate neutrophil mobilization, recruitment and activation.


Similar content being viewed by others
References
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316(8):1324–1331. doi:10.1016/j.yexcr.2010.02.045
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. doi:10.1038/nrc3245
Wels J, Kaplan RN, Rafii S, Lyden D (2008) Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev 22(5):559–574. doi:10.1101/gad.1636908
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL (2005) Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res 65(19):8896–8904. doi:10.1158/0008-5472.CAN-05-1734
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. doi:10.1038/nature01322
Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S (2013) Tumor associated macrophages and neutrophils in cancer. Immunobiology. doi:10.1016/j.imbio.2013.06.003
Souto JC, Vila L, Bru A (2011) Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Med Res Rev 31(3):311–363. doi:10.1002/med.20185
Tazzyman S, Lewis CE, Murdoch C (2009) Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol 90(3):222–231. doi:10.1111/j.1365-2613.2009.00641.x
Gregory AD, Houghton AM (2011) Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 71(7):2411–2416. doi:10.1158/0008-5472.CAN-10-2583
Fridlender ZG, Albelda SM (2012) Tumor-associated neutrophils: friend or foe? Carcinogenesis 33(5):949–955. doi:10.1093/carcin/bgs123
Brandau S, Dumitru CA, Lang S (2013) Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol 35(2):163–176. doi:10.1007/s00281-012-0344-6
Fioretti F, Fradelizi D, Stoppacciaro A, Ramponi S, Ruco L, Minty A, Sozzani S, Garlanda C, Vecchi A, Mantovani A (1998) Reduced tumorigenicity and augmented leukocyte infiltration after monocyte chemotactic protein-3 (MCP-3) gene transfer: perivascular accumulation of dendritic cells in peritumoral tissue and neutrophil recruitment within the tumor. J Immunol 161(1):342–346
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16(3):183–194. doi:10.1016/j.ccr.2009.06.017
Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo MP (1993) Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med 178(1):151–161
Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11(8):519–531. doi:10.1038/nri3024
Cavallo F, Giovarelli M, Gulino A, Vacca A, Stoppacciaro A, Modesti A, Forni G (1992) Role of neutrophils and CD4+ T lymphocytes in the primary and memory response to nonimmunogenic murine mammary adenocarcinoma made immunogenic by IL-2 gene. J Immunol 149(11):3627–3635
Suttmann H, Riemensberger J, Bentien G, Schmaltz D, Stockle M, Jocham D, Bohle A, Brandau S (2006) Neutrophil granulocytes are required for effective Bacillus Calmette-Guerin immunotherapy of bladder cancer and orchestrate local immune responses. Cancer Res 66(16):8250–8257. doi:10.1158/0008-5472.CAN-06-1416
Kousis PC, Henderson BW, Maier PG, Gollnick SO (2007) Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res 67(21):10501–10510. doi:10.1158/0008-5472.CAN-07-1778
Midorikawa Y, Yamashita T, Sendo F (1990) Modulation of the immune response to transplanted tumors in rats by selective depletion of neutrophils in vivo using a monoclonal antibody: abrogation of specific transplantation resistance to chemical carcinogen-induced syngeneic tumors by selective depletion of neutrophils in vivo. Cancer Res 50(19):6243–6247
Ben-Neriah Y, Karin M (2011) Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 12(8):715–723. doi:10.1038/ni.2060
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. doi:10.1126/science.1203486
Piccard H, Muschel RJ, Opdenakker G (2012) On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 82(3):296–309. doi:10.1016/j.critrevonc.2011.06.004
Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C (1999) Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res 5(6):1369–1379
Chen Z, Colon I, Ortiz N, Callister M, Dong G, Pegram MY, Arosarena O, Strome S, Nicholson JC, Van Waes C (1998) Effects of interleukin-1alpha, interleukin-1 receptor antagonist, and neutralizing antibody on proinflammatory cytokine expression by human squamous cell carcinoma lines. Cancer Res 58(16):3668–3676
Arii K, Tanimura H, Iwahashi M, Tsunoda T, Tani M, Noguchi K, Mizobata S, Hotta T, Nakamori M, Yamaue H (2000) Neutrophil functions and cytokine production in patients with gastric cancer. Hepatogastroenterology 47(31):291–297
Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJ, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N (2010) Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A 107(50):21248–21255. doi:10.1073/pnas.1015855107
Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111(12):5457–5466. doi:10.1182/blood-2008-01-136895
Fridlender ZG, Buchlis G, Kapoor V, Cheng G, Sun J, Singhal S, Crisanti MC, Wang LC, Heitjan D, Snyder LA, Albelda SM (2010) CCL2 blockade augments cancer immunotherapy. Cancer Res 70(1):109–118. doi:10.1158/0008-5472.CAN-09-2326
Ninck S, Reisser C, Dyckhoff G, Helmke B, Bauer H, Herold-Mende C (2003) Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer 106(1):34–44. doi:10.1002/ijc.11188
Lin WW, Karin M (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 117(5):1175–1183. doi:10.1172/JCI31537
Revoltella RP, Menicagli M, Campani D (2012) Granulocyte-macrophage colony-stimulating factor as an autocrine survival-growth factor in human gliomas. Cytokine 57(3):347–359. doi:10.1016/j.cyto.2011.11.016
Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R (2011) Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20(3):300–314. doi:10.1016/j.ccr.2011.08.012
Forte G, Sorrentino R, Montinaro A, Luciano A, Adcock IM, Maiolino P, Arra C, Cicala C, Pinto A, Morello S (2012) Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol 189(5):2226–2233. doi:10.4049/jimmunol.1200744
Kandasamy M, Bay BH, Lee YK, Mahendran R (2011) Lactobacilli secreting a tumor antigen and IL15 activates neutrophils and dendritic cells and generates cytotoxic T lymphocytes against cancer cells. Cell Immunol 271(1):89–96. doi:10.1016/j.cellimm.2011.06.004
Schwandt A, Garcia JA, Elson P, Wyckhouse J, Finke JH, Ireland J, Triozzi P, Zhou M, Dreicer R, Rini BI (2011) Clinical and immunomodulatory effects of celecoxib plus interferon-alpha in metastatic renal cell carcinoma patients with COX-2 tumor immunostaining. J Clin Immunol 31(4):690–698. doi:10.1007/s10875-011-9530-x
Yamamoto M, Kamigaki T, Yamashita K, Hori Y, Hasegawa H, Kuroda D, Moriyama H, Nagata M, Ku Y, Kuroda Y (2009) Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep 22(2):337–343
de Visser KE, Coussens LM (2006) The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol 13:118–137. doi:10.1159/000092969
Kenny PA, Bissell MJ (2003) Tumor reversion: correction of malignant behavior by microenvironmental cues. Int J Cancer 107(5):688–695. doi:10.1002/ijc.11491
Noguera R, Nieto OA, Tadeo I, Farinas F, Alvaro T (2012) Extracellular matrix, biotensegrity and tumor microenvironment. An update and overview. Histol Histopathol 27(6):693–705
Mocellin S, Rossi CR, Pilati P, Nitti D (2005) Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev 16(1):35–53. doi:10.1016/j.cytogfr.2004.11.001
Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F (1999) Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med 5(7):828–831. doi:10.1038/10552
Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J (2011) IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 10:150. doi:10.1186/1476-4598-10-150
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M (2004) Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 6(3):297–305. doi:10.1016/j.ccr.2004.08.012
Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC (2008) TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood 111(10):4997–5007. doi:10.1182/blood-2007-08-108597
Jing Y, Ma N, Fan T, Wang C, Bu X, Jiang G, Li R, Gao L, Li D, Wu M, Wei L (2011) Tumor necrosis factor-alpha promotes tumor growth by inducing vascular endothelial growth factor. Cancer Invest 29(7):485–493. doi:10.3109/07357907.2011.597812
Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH (2013) Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol 85(4):531–540. doi:10.1016/j.bcp.2012.11.021
Whalen GF (1990) Solid tumours and wounds: transformed cells misunderstood as injured tissue? Lancet 336(8729):1489–1492
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795. doi:10.1172/JCI59643
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM (2008) Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14(11):1835–1842. doi:10.1089/ten.tea.2007.0264
Mantovani A, Locati M (2013) Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol 33(7):1478–1483. doi:10.1161/ATVBAHA.113.300168
Hargadon KM (2013) Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol 4:192. doi:10.3389/fimmu.2013.00192
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107(5):2112–2122. doi:10.1182/blood-2005-01-0428
Kuang DM, Peng C, Zhao Q, Wu Y, Zhu LY, Wang J, Yin XY, Li L, Zheng L (2010) Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol 185(3):1544–1549. doi:10.4049/jimmunol.0904094
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W (2011) Th17 cells in cancer: help or hindrance? Carcinogenesis 32(5):643–649. doi:10.1093/carcin/bgr019
Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A (1999) Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol 162(4):2347–2352
Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L (2011) Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol 54(5):948–955. doi:10.1016/j.jhep.2010.08.041
Ley K, Smith E, Stark MA (2006) IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res 34(3):229–242. doi:10.1385/IR:34:3:229
Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22(3):285–294. doi:10.1016/j.immuni.2005.01.011
Houghton AM (2010) The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 9(9):1732–1737
Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA (2000) The neutrophil as a cellular source of chemokines. Immunol Rev 177:195–203
Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115(2):335–343. doi:10.1182/blood-2009-04-216085
Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA (2007) An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol 179(11):7791–7799
Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S (2010) Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 120(4):1151–1164. doi:10.1172/JCI37223
Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM (2012) Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One 7(2):e31524. doi:10.1371/journal.pone.0031524
Lopez-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS (2013) Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene 32(14):1752–1760. doi:10.1038/onc.2012.201
Swann JB, Smyth MJ (2007) Immune surveillance of tumors. J Clin Invest 117(5):1137–1146. doi:10.1172/JCI31405
Zhang Q, Yang X, Pins M, Javonovic B, Kuzel T, Kim SJ, Parijs LV, Greenberg NM, Liu V, Guo Y, Lee C (2005) Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer Res 65(5):1761–1769. doi:10.1158/0008-5472.CAN-04-3169
Gholamin M, Moaven O, Memar B, Farshchian M, Naseh H, Malekzadeh R, Sotoudeh M, Rajabi-Mashhadi MT, Forghani MN, Farrokhi F, Abbaszadegan MR (2009) Overexpression and interactions of interleukin-10, transforming growth factor beta, and vascular endothelial growth factor in esophageal squamous cell carcinoma. World J Surg 33(7):1439–1445. doi:10.1007/s00268-009-0070-y
Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP (1999) Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 5(10):2963–2970
Gerlini G, Tun-Kyi A, Dudli C, Burg G, Pimpinelli N, Nestle FO (2004) Metastatic melanoma secreted IL-10 down-regulates CD1 molecules on dendritic cells in metastatic tumor lesions. Am J Pathol 165(6):1853–1863. doi:10.1016/S0002-9440(10)63238-5
Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, Cannan D, Ramacher M, Kato M, Overwijk WW, Chen SH, Umansky VY, Sikora AG (2012) Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol 188(11):5365–5376. doi:10.4049/jimmunol.1103553
Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V (2013) Nitric oxide and cancer: a review. World J Surg Oncol 11:118. doi:10.1186/1477-7819-11-118
Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30(3):377–386. doi:10.1093/carcin/bgp014
Brown JR, DuBois RN (2005) COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol 23(12):2840–2855. doi:10.1200/JCO.2005.09.051
McKallip R, Li R, Ladisch S (1999) Tumor gangliosides inhibit the tumor-specific immune response. J Immunol 163(7):3718–3726
Hossain DM, Mohanty S, Ray P, Das T, Sa G (2012) Tumor gangliosides and T cells: a deadly encounter. Front Biosci (Schol Ed) 4:502–519
Bierie B, Moses HL (2010) Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 21(1):49–59. doi:10.1016/j.cytogfr.2009.11.008
Wojtowicz-Praga S (2003) Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs 21(1):21–32
Levy L, Hill CS (2006) Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev 17(1–2):41–58. doi:10.1016/j.cytogfr.2005.09.009
Barcellos-Hoff MH, Dix TA (1996) Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10(9):1077–1083
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13(1):23–35. doi:10.1016/j.ccr.2007.12.004
Zamarron BF, Chen W (2011) Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 7(5):651–658
Lechner MG, Megiel C, Russell SM, Bingham B, Arger N, Woo T, Epstein AL (2011) Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med 9:90. doi:10.1186/1479-5876-9-90
Shen L, Smith JM, Shen Z, Eriksson M, Sentman C, Wira CR (2007) Inhibition of human neutrophil degranulation by transforming growth factor-beta1. Clin Exp Immunol 149(1):155–161. doi:10.1111/j.1365-2249.2007.03376.x
Malipiero U, Koedel U, Pfister HW, Leveen P, Burki K, Reith W, Fontana A (2006) TGFbeta receptor II gene deletion in leucocytes prevents cerebral vasculitis in bacterial meningitis. Brain 129(Pt 9):2404–2415. doi:10.1093/brain/awl192
Parekh T, Saxena B, Reibman J, Cronstein BN, Gold LI (1994) Neutrophil chemotaxis in response to TGF-beta isoforms (TGF-beta 1, TGF-beta 2, TGF-beta 3) is mediated by fibronectin. J Immunol 152(5):2456–2466
Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, Lucas C, Townes AS (1991) Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med 173(5):1121–1132
Reibman J, Meixler S, Lee TC, Gold LI, Cronstein BN, Haines KA, Kolasinski SL, Weissmann G (1991) Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc Natl Acad Sci U S A 88(15):6805–6809
Kim S, Buchlis G, Fridlender ZG, Sun J, Kapoor V, Cheng G, Haas A, Cheung HK, Zhang X, Corbley M, Kaiser LR, Ling L, Albelda SM (2008) Systemic blockade of transforming growth factor-beta signaling augments the efficacy of immunogene therapy. Cancer Res 68(24):10247–10256. doi:10.1158/0008-5472.CAN-08-1494
Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR (1996) Transforming growth factor-beta 1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol 157(1):360–368
Bierie B, Stover DG, Abel TW, Chytil A, Gorska AE, Aakre M, Forrester E, Yang L, Wagner KU, Moses HL (2008) Transforming growth factor-beta regulates mammary carcinoma cell survival and interaction with the adjacent microenvironment. Cancer Res 68(6):1809–1819. doi:10.1158/0008-5472.CAN-07-5597
Kalinski P (2012) Regulation of immune responses by prostaglandin E2. J Immunol 188(1):21–28. doi:10.4049/jimmunol.1101029
Wang MT, Honn KV, Nie D (2007) Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev 26(3–4):525–534. doi:10.1007/s10555-007-9096-5
Eisinger AL, Prescott SM, Jones DA, Stafforini DM (2007) The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins Other Lipid Mediat 82(1–4):147–154. doi:10.1016/j.prostaglandins.2006.05.026
Schrey MP, Patel KV (1995) Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. Br J Cancer 72(6):1412–1419
Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, Joensuu H, Isola J (2002) Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res 62(3):632–635
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65(8):3044–3048. doi:10.1158/0008-5472.CAN-04-4505
Riedl K, Krysan K, Pold M, Dalwadi H, Heuze-Vourc’h N, Dohadwala M, Liu M, Cui X, Figlin R, Mao JT, Strieter R, Sharma S, Dubinett SM (2004) Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat 7(3):169–184. doi:10.1016/j.drup.2004.04.003
Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P (2012) PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Investig 41(6–7):635–657. doi:10.3109/08820139.2012.695417
Plescia OJ, Smith AH, Grinwich K (1975) Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A 72(5):1848–1851
Sionov RV, Gallily R (1990) Engulfment and intracellular killing of F9 teratocarcinoma cells by non-activated murine macrophages. Int Immunol 2(4):291–301
Mulligan JK, Rosenzweig SA, Young MR (2010) Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J Immunother 33(2):126–135. doi:10.1097/CJI.0b013e3181b91c9c
Khayrullina T, Yen JH, Jing H, Ganea D (2008) In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol 181(1):721–735
Lemos HP, Grespan R, Vieira SM, Cunha TM, Verri WA Jr, Fernandes KS, Souto FO, McInnes IB, Ferreira SH, Liew FY, Cunha FQ (2009) Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proc Natl Acad Sci U S A 106(14):5954–5959. doi:10.1073/pnas.0812782106
Karavitis J, Zhang M (2013) COX2 regulation of breast cancer bone metastasis. Oncoimmunology 2(3):e23129. doi:10.4161/onci.23129
Yu Y, Chadee K (1998) Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J Immunol 161(7):3746–3752
Nakayama T, Mutsuga N, Yao L, Tosato G (2006) Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukoc Biol 79(1):95–104. doi:10.1189/jlb.0405226
Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, Wilson MS, Taylor GW, Jose PJ, Williams TJ (2007) Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc Natl Acad Sci U S A 104(28):11712–11717. doi:10.1073/pnas.0701700104
Aso H, Ito S, Mori A, Morioka M, Suganuma N, Kondo M, Imaizumi K, Hasegawa Y (2012) Prostaglandin E2 enhances interleukin-8 production via EP4 receptor in human pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 302(2):L266–L273. doi:10.1152/ajplung.00248.2011
He LK, Liu LH, Hahn E, Gamelli RL (2001) The expression of cyclooxygenase and the production of prostaglandin E2 in neutrophils after burn injury and infection. J Burn Care Rehabil 22(1):58–64
Cockeran R, Steel HC, Mitchell TJ, Feldman C, Anderson R (2001) Pneumolysin potentiates production of prostaglandin E(2) and leukotriene B(4) by human neutrophils. Infect Immun 69(5):3494–3496. doi:10.1128/IAI.69.5.3494-3496.2001
Ottonello L, Gonella R, Dapino P, Sacchetti C, Dallegri F (1998) Prostaglandin E2 inhibits apoptosis in human neutrophilic polymorphonuclear leukocytes: role of intracellular cyclic AMP levels. Exp Hematol 26(9):895–902
Sottile A, Venza M, Venza I, Teti D (1995) Prostaglandins affect the respiratory burst of human neutrophils. Immunopharmacol Immunotoxicol 17(2):311–321. doi:10.3109/08923979509019753
Ham EA, Soderman DD, Zanetti ME, Dougherty HW, McCauley E, Kuehl FA Jr (1983) Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc Natl Acad Sci U S A 80(14):4349–4353
Talpain E, Armstrong RA, Coleman RA, Vardey CJ (1995) Characterization of the PGE receptor subtype mediating inhibition of superoxide production in human neutrophils. Br J Pharmacol 114(7):1459–1465
Ottonello L, Morone MP, Dapino P, Dallegri F (1995) Cyclic AMP-elevating agents down-regulate the oxidative burst induced by granulocyte-macrophage colony-stimulating factor (GM-CSF) in adherent neutrophils. Clin Exp Immunol 101(3):502–506
Yu CL, Huang MH, Kung YY, Tsai CY, Tsai YY, Tsai ST, Huang DF, Sun KH, Han SH, Yu HS (1998) Interleukin-13 increases prostaglandin E2 (PGE2) production by normal human polymorphonuclear neutrophils by enhancing cyclooxygenase 2 (COX-2) gene expression. Inflamm Res 47(4):167–173
Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM (2000) Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol 164(1):361–370
Chan AT, Cook NR (2012) Are we ready to recommend aspirin for cancer prevention? Lancet 379(9826):1569–1571. doi:10.1016/S0140-6736(11)61654-1
Dominguez-Luis M, Herrera-Garcia A, Arce-Franco M, Armas-Gonzalez E, Rodriguez-Pardo M, Lorenzo-Diaz F, Feria M, Cadenas S, Sanchez-Madrid F, Diaz-Gonzalez F (2013) Superoxide anion mediates the L-selectin down-regulation induced by non-steroidal anti-inflammatory drugs in human neutrophils. Biochem Pharmacol 85(2):245–256. doi:10.1016/j.bcp.2012.10.024
Baratelli F, Lee JM, Hazra S, Lin Y, Walser TC, Schaue D, Pak PS, Elashoff D, Reckamp K, Zhang L, Fishbein MC, Sharma S, Dubinett SM (2010) PGE(2) contributes to TGF-beta induced T regulatory cell function in human non-small cell lung cancer. Am J Transl Res 2(4):356–367
Godin-Ethier J, Hanafi LA, Piccirillo CA, Lapointe R (2011) Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res 17(22):6985–6991. doi:10.1158/1078-0432.CCR-11-1331
Katz JB, Muller AJ, Prendergast GC (2008) Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 222:206–221. doi:10.1111/j.1600-065X.2008.00610.x
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In’t Veld P, De Baetselier P, Van Ginderachter JA (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70(14):5728–5739. doi:10.1158/0008-5472.CAN-09-4672
Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X (2013) Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 190(7):3783–3797. doi:10.4049/jimmunol.1201449
Blache CA, Manuel ER, Kaltcheva TI, Wong AN, Ellenhorn JD, Blazar BR, Diamond DJ (2012) Systemic delivery of Salmonella typhimurium transformed with IDO shRNA enhances intratumoral vector colonization and suppresses tumor growth. Cancer Res 72(24):6447–6456. doi:10.1158/0008-5472.CAN-12-0193
Whiteside TL, Jackson EK (2013) Adenosine and prostaglandin E2 production by human inducible regulatory T cells in health and disease. Front Immunol 4:212. doi:10.3389/fimmu.2013.00212
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204(6):1257–1265. doi:10.1084/jem.20062512
Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2(2):351–360. doi:10.1007/s11302-005-5302-5
Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K, Coe A, Kincade PW, Thompson LF (1998) CD73 expression and fyn-dependent signaling on murine lymphocytes. Eur J Immunol 28(10):2981–2990. doi:10.1002/(SICI)1521-4141(199810)28:10<2981::AID-IMMU2981>3.0.CO;2-D
Zhang B (2010) CD73: a novel target for cancer immunotherapy. Cancer Res 70(16):6407–6411. doi:10.1158/0008-5472.CAN-10-1544
Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, Shin T, Curiel TJ, Zhang B (2010) CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res 70(6):2245–2255. doi:10.1158/0008-5472.CAN-09-3109
Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK (2009) Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J 23(2):473–482. doi:10.1096/fj.08-119701
Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, Smyth MJ (2011) CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71(8):2892–2900. doi:10.1158/0008-5472.CAN-10-4246
Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ (2010) Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 107(4):1547–1552. doi:10.1073/pnas.0908801107
Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI (2001) Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 166(1):678–689
An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ (2010) Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 15(6):516–522. doi:10.3109/1354750X.2010.491557
Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F (2013) Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer 108(10):2116–2122. doi:10.1038/bjc.2013.167
Chen TM, Lin CC, Huang PT, Wen CF (2012) Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol 27(3):553–561. doi:10.1111/j.1440-1746.2011.06910.x
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, Lee K (2009) Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 58(1):15–23. doi:10.1007/s00262-008-0516-3
Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS (2012) CD15+/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol 33(1):121–129. doi:10.1007/s13277-011-0254-6
Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M (2013) Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One 8(3):e58184. doi:10.1371/journal.pone.0058184
Demirtas A, Sabur V, Akinsal EC, Demirci D, Ekmekcioglu O, Gulmez I, Tatlisen A (2013) Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy? ScientificWorldJournal 2013:703579. doi:10.1155/2013/703579
Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, Wan DS, Pan ZZ (2010) Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 25(12):1427–1433. doi:10.1007/s00384-010-1052-0
Dumitru CA, Lang S, Brandau S (2013) Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol 23(3):141–148. doi:10.1016/j.semcancer.2013.02.005
Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S (2012) Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer 130(5):1109–1119. doi:10.1002/ijc.26123
Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR (2008) Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 32(8):1757–1762. doi:10.1007/s00268-008-9552-6
Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M (2012) Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 79(5):1085–1091. doi:10.1016/j.urology.2011.11.070
Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 34(1):55–60. doi:10.1016/j.ejso.2007.02.014
Jin H, Zhang G, Liu X, Chen C, Yu H, Huang X, Zhang Q, Yu J (2013) Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol 11:112. doi:10.1186/1477-7819-11-112
Liu H, Liu G, Bao Q, Sun W, Bao H, Bi L, Wen W, Liu Y, Wang Z, Yin X, Bai Y, Hu X (2010) The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in rectal carcinoma. J Gastrointest Cancer 41(2):116–120. doi:10.1007/s12029-009-9125-4
Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M (2012) Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol 187(2):411–417. doi:10.1016/j.juro.2011.10.026
Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E (2009) Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 137(2):425–428. doi:10.1016/j.jtcvs.2008.05.046
Sato T, Omura M, Saito J, Hirasawa A, Kakuta Y, Wakabayashi Y, Nishikawa T (2000) Neutrophilia associated with anaplastic carcinoma of the thyroid: production of macrophage colony-stimulating factor (M-CSF) and interleukin-6. Thyroid 10(12):1113–1118
Schmidt H, Suciu S, Punt CJ, Gore M, Kruit W, Patel P, Lienard D, von der Maase H, Eggermont AM, Keilholz U (2007) Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 25(12):1562–1569. doi:10.1200/JCO.2006.09.0274
Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, Scherag A, Hutte J, Dominas N, Lehnerdt GF, Hoffmann TK, Lang S, Brandau S (2011) Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer 129(9):2183–2193. doi:10.1002/ijc.25892
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91(3):181–184. doi:10.1002/jso.20329
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M (2007) The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 73(3–4):215–220. doi:10.1159/000127412
Melani C, Chiodoni C, Forni G, Colombo MP (2003) Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102(6):2138–2145. doi:10.1182/blood-2003-01-0190
Kusmartsev S, Gabrilovich DI (2006) Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother 55(3):237–245. doi:10.1007/s00262-005-0048-z
Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D (2003) All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63(15):4441–4449
Liu H, Tabuchi T, Takemura A, Kasuga T, Motohashi G, Hiraishi K, Katano M, Nakada I, Ubukata H (2008) The granulocyte/lymphocyte ratio as an independent predictor of tumour growth, metastasis and progression: its clinical applications. Mol Med Rep 1(5):699–704. doi:10.3892/mmr_00000016
Aeed PA, Nakajima M, Welch DR (1988) The role of polymorphonuclear leukocytes (PMN) on the growth and metastatic potential of 13762NF mammary adenocarcinoma cells. Int J Cancer 42(5):748–759
McGary CT, Miele ME, Welch DR (1995) Highly metastatic 13762NF rat mammary adenocarcinoma cell clones stimulate bone marrow by secretion of granulocyte-macrophage colony-stimulating factor/interleukin-3 activity. Am J Pathol 147(6):1668–1681
Welch DR, Schissel DJ, Howrey RP, Aeed PA (1989) Tumor-elicited polymorphonuclear cells, in contrast to “normal” circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A 86(15):5859–5863
Opdenakker G, Van Damme J (2004) The countercurrent principle in invasion and metastasis of cancer cells. Recent insights on the roles of chemokines. Int J Dev Biol 48(5–6):519–527. doi:10.1387/ijdb.041796go
Schaider H, Oka M, Bogenrieder T, Nesbit M, Satyamoorthy K, Berking C, Matsushima K, Herlyn M (2003) Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. Int J Cancer 103(3):335–343. doi:10.1002/ijc.10775
Lee LF, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K, Ting JP (2000) IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration. J Immunol 164(5):2769–2775
Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J (1998) Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol 152(1):83–92
Gijsbers K, Gouwy M, Struyf S, Wuyts A, Proost P, Opdenakker G, Penninckx F, Ectors N, Geboes K, Van Damme J (2005) GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp Cell Res 303(2):331–342. doi:10.1016/j.yexcr.2004.09.027
Asano Y, Yokoyama T, Shibata S, Kobayashi S, Shimoda K, Nakashima H, Okamura S, Niho Y (1997) Effect of the chimeric soluble granulocyte colony-stimulating factor receptor on the proliferation of leukemic blast cells from patients with acute myeloblastic leukemia. Cancer Res 57(16):3395–3397
Chakraborty A, Guha S (2007) Granulocyte colony-stimulating factor/granulocyte colony-stimulating factor receptor biological axis promotes survival and growth of bladder cancer cells. Urology 69(6):1210–1215. doi:10.1016/j.urology.2007.02.035
Joshita S, Nakazawa K, Sugiyama Y, Kamijo A, Matsubayashi K, Miyabayashi H, Furuta K, Kitano K, Kawa S (2009) Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern Med 48(9):687–691
Kyo S, Kanaya T, Takakura M, Inoue M (2000) A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and Il-6. Gynecol Oncol 78(3 Pt 1):383–387. doi:10.1006/gyno.2000.5904
Savarese TM, Mitchell K, McQuain C, Campbell CL, Guardiani R, Wuu J, Ollari C, Reale F, Nelson BE, Chen A, Quesenberry PJ (2001) Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett 162(1):105–115
Tsukuda M, Nagahara T, Yago T, Matsuda H, Yanoma S (1993) Production of granulocyte colony-stimulating factor by head and neck carcinomas. Biotherapy 6(3):183–187
Liu H, Zhang Z, Tabuchi T, Wang S, Wang J (2013) The role of pro-inflammatory cytokines and immune cells in colorectal carcinoma progression. Oncol Lett 5(4):1177–1182. doi:10.3892/ol.2013.1176
Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G (2007) Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9(1):R15. doi:10.1186/bcr1648
Urdinguio RG, Fernandez AF, Moncada-Pazos A, Huidobro C, Rodriguez RM, Ferrero C, Martinez-Camblor P, Obaya AJ, Bernal T, Parra-Blanco A, Rodrigo L, Santacana M, Matias-Guiu X, Soldevilla B, Dominguez G, Bonilla F, Cal S, Lopez-Otin C, Fraga MF (2013) Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res 73(1):395–405. doi:10.1158/0008-5472.CAN-12-0806
Braun B, Lange M, Oeckler R, Mueller MM (2004) Expression of G-CSF and GM-CSF in human meningiomas correlates with increased tumor proliferation and vascularization. J Neurooncol 68(2):131–140
Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L (2007) Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol 18(2):226–232. doi:10.1093/annonc/mdl158
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. doi:10.1200/JCO.2006.08.5829
Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP (1999) Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 162(10):5728–5737
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I (2004) High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res 64(17):6337–6343. doi:10.1158/0008-5472.CAN-04-0757
Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM (1990) Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res 50(2):227–234
Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC (1993) Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A 90(8):3539–3543
Avalos BR, Gasson JC, Hedvat C, Quan SG, Baldwin GC, Weisbart RH, Williams RE, Golde DW, DiPersio JF (1990) Human granulocyte colony-stimulating factor: biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines. Blood 75(4):851–857
Kobashi Y, Okimoto N, Sakamoto K (2004) Squamous cell carcinoma of the lung producing granulocyte colony-stimulating factor and resembling a malignant pleural mesothelioma. Intern Med 43(2):111–116
Hsu DM, Agarwal S, Benham A, Coarfa C, Trahan DN, Chen Z, Stowers PN, Courtney AN, Lakoma A, Barbieri E, Metelitsa LS, Gunaratne P, Kim ES, Shohet JM (2013) G-CSF receptor positive neuroblastoma subpopulations are enriched in chemotherapy-resistant or relapsed tumors and are highly tumorigenic. Cancer Res 73(13):4134–4146. doi:10.1158/0008-5472.CAN-12-4056
Panopoulos AD, Watowich SS (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42(3):277–288. doi:10.1016/j.cyto.2008.03.002
Abrams SI, Waight JD (2012) Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology 1(4):550–551
Waight JD, Hu Q, Miller A, Liu S, Abrams SI (2011) Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One 6(11):e27690. doi:10.1371/journal.pone.0027690
Tachibana M, Murai M (1998) G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther 4(2):113–120
Tachibana M, Miyakawa A, Uchida A, Murai M, Eguchi K, Nakamura K, Kubo A, Hata JI (1997) Granulocyte colony-stimulating factor receptor expression on human transitional cell carcinoma of the bladder. Br J Cancer 75(10):1489–1496
Ninci EB, Brandstetter T, Meinhold-Heerlein I, Bettendorf H, Sellin D, Bauknecht T (2000) G-CSF receptor expression in ovarian cancer. Int J Gynecol Cancer 10(1):19–26
Yang X, Liu F, Xu Z, Chen C, Wu X, Li G, Li J (2005) Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J 81(955):333–337. doi:10.1136/pgmj.2004.024646
Morales-Arias J, Meyers PA, Bolontrade MF, Rodriguez N, Zhou Z, Reddy K, Chou AJ, Koshkina NV, Kleinerman ES (2007) Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer 110(7):1568–1577. doi:10.1002/cncr.22964
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C (2002) Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol 15(8):831–837. doi:10.1097/01.MP.0000020391.98998.6B
Griffiths AP, Rice A, Dixon MF (1998) Anaplastic gastric adenocarcinoma with extensive neutrophilic infiltration. Histopathology 33(4):392–393
Rice AJ, Griffiths AP, Martin IG, Dixon MF (2000) Gastric carcinoma with prominent neutrophil infiltration. Histopathology 37(3):289–290
Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, Antoine M, Soler P, Cadranel J (2003) Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res 63(6):1405–1412
Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, Mouroux J, Hofman P (2012) Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 118(6):1726–1737. doi:10.1002/cncr.26456
Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V (2011) Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol 24(12):1612–1619. doi:10.1038/modpathol.2011.113
Gaida MM, Steffen TG, Gunther F, Tschaharganeh DF, Felix K, Bergmann F, Schirmacher P, Hansch GM (2012) Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. Eur J Immunol 42(12):3369–3380. doi:10.1002/eji.201242628
Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML (1999) Neutrophil infiltration into human gliomas. Acta Neuropathol 98(4):349–354
O’Sullivan C, Lewis CE (1994) Tumour-associated leucocytes: friends or foes in breast carcinoma. J Pathol 172(3):229–235. doi:10.1002/path.1711720302
Ancrile BB, O’Hayer KM, Counter CM (2008) Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Mol Interv 8(1):22–27. doi:10.1124/mi.8.1.6
Sparmann A, Bar-Sagi D (2004) Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6(5):447–458. doi:10.1016/j.ccr.2004.09.028
Lazennec G, Richmond A (2010) Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 16(3):133–144. doi:10.1016/j.molmed.2010.01.003
Wuyts A, Proost P, Lenaerts JP, Ben-Baruch A, Van Damme J, Wang JM (1998) Differential usage of the CXC chemokine receptors 1 and 2 by interleukin-8, granulocyte chemotactic protein-2 and epithelial-cell-derived neutrophil attractant-78. Eur J Biochem 255(1):67–73
Ahuja SK, Murphy PM (1996) The CXC chemokines growth-regulated oncogene (GRO) alpha, GRObeta, GROgamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem 271(34):20545–20550
Song J, Wu C, Zhang X, Sorokin LM (2013) In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1beta-induced peritonitis. J Immunol 190(1):401–410. doi:10.4049/jimmunol.1202286
Schenk BI, Petersen F, Flad HD, Brandt E (2002) Platelet-derived chemokines CXC chemokine ligand (CXCL)7, connective tissue-activating peptide III, and CXCL4 differentially affect and cross-regulate neutrophil adhesion and transendothelial migration. J Immunol 169(5):2602–2610
Bru A, Souto JC, Alcolea S, Anton R, Remacha A, Camacho M, Soler M, Bru I, Porres A, Vila L (2009) Tumour cell lines HT-29 and FaDu produce proinflammatory cytokines and activate neutrophils in vitro: possible applications for neutrophil-based antitumour treatment. Mediators Inflamm 2009:817498. doi:10.1155/2009/817498
Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T (2001) Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukoc Biol 69(5):698–704
Pham CT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6(7):541–550. doi:10.1038/nri1841
Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ, Sansom OJ (2012) Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest 122(9):3127–3144. doi:10.1172/JCI61067
Verbeke H, Struyf S, Berghmans N, Van Coillie E, Opdenakker G, Uyttenhove C, Van Snick J, Van Damme J (2011) Isotypic neutralizing antibodies against mouse GCP-2/CXCL6 inhibit melanoma growth and metastasis. Cancer Lett 302(1):54–62. doi:10.1016/j.canlet.2010.12.013
Richards H, Williams A, Jones E, Hindley J, Godkin A, Simon AK, Gallimore A (2010) Novel role of regulatory T cells in limiting early neutrophil responses in skin. Immunology 131(4):583–592. doi:10.1111/j.1365-2567.2010.03333.x
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7(11):1194–1201. doi:10.1038/nm1101-1194
Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E (2006) VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124(1):175–189. doi:10.1016/j.cell.2005.10.036
Kusmartsev S, Gabrilovich DI (2006) Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev 25(3):323–331. doi:10.1007/s10555-006-9002-6
Ji YN, Wang Q, Li Y, Wang Z (2013) Prognostic value of vascular endothelial growth factor A expression in gastric cancer: a meta-analysis. Tumour Biol. doi:10.1007/s13277-013-1371-1
Minardi D, Lucarini G, Santoni M, Mazzucchelli R, Burattini L, Pistelli M, Bianconi M, Di Primio R, Scartozzi M, Montironi R, Cascinu S, Muzzonigro G (2013) VEGF expression and response to sunitinib in patients with metastatic clear cell renal cell carcinoma. Anticancer Res 33(11):5017–5022
Ohm JE, Carbone DP (2001) VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res 23(2–3):263–272. doi:10.1385/IR:23:2-3:263
Salmaggi A, Gelati M, Pollo B, Frigerio S, Eoli M, Silvani A, Broggi G, Ciusani E, Croci D, Boiardi A, De Rossi M (2004) CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. J Neurooncol 67(3):305–317
Santiago B, Calonge E, Del Rey MJ, Gutierrez-Canas I, Izquierdo E, Usategui A, Galindo M, Alcami J, Pablos JL (2011) CXCL12 gene expression is upregulated by hypoxia and growth arrest but not by inflammatory cytokines in rheumatoid synovial fibroblasts. Cytokine 53(2):184–190. doi:10.1016/j.cyto.2010.06.006
Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W (2005) CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res 65(2):465–472
Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, Ouyang W, Ferrara N (2013) An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. doi:10.1038/nm.3291
Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31(5):787–798. doi:10.1016/j.immuni.2009.09.014
Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF (2013) Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 190(5):2415–2423. doi:10.4049/jimmunol.1202535
Mantovani A, Sica A (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 22(2):231–237. doi:10.1016/j.coi.2010.01.009
Bailey C, Negus R, Morris A, Ziprin P, Goldin R, Allavena P, Peck D, Darzi A (2007) Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin Exp Metastasis 24(2):121–130. doi:10.1007/s10585-007-9060-3
Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M (2001) Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92(5):1085–1091. doi:10.1002/1097-0142(20010901)92:5<1085::AID-CNCR1424>3.0.CO;2-K
Tanaka K, Kurebayashi J, Sohda M, Nomura T, Prabhakar U, Yan L, Sonoo H (2009) The expression of monocyte chemotactic protein-1 in papillary thyroid carcinoma is correlated with lymph node metastasis and tumor recurrence. Thyroid 19(1):21–25. doi:10.1089/thy.2008.0237
Yoshidome H, Kohno H, Shida T, Kimura F, Shimizu H, Ohtsuka M, Nakatani Y, Miyazaki M (2009) Significance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastases. Int J Oncol 34(4):923–930
Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A (2006) The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol 208(4):507–517. doi:10.1002/path.1918
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475(7355):222–225. doi:10.1038/nature10138. nature10138
Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH (2007) CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett 252(1):86–92. doi:10.1016/j.canlet.2006.12.012
Nakashima E, Mukaida N, Kubota Y, Kuno K, Yasumoto K, Ichimura F, Nakanishi I, Miyasaka M, Matsushima K (1995) Human MCAF gene transfer enhances the metastatic capacity of a mouse cachectic adenocarcinoma cell line in vivo. Pharm Res 12(11):1598–1604
Zhang J, Lu Y, Pienta KJ (2010) Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst 102(8):522–528. doi:10.1093/jnci/djq044
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ (2007) Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 67(19):9417–9424
Yang EJ, Choi E, Ko J, Kim DH, Lee JS, Kim IS (2012) Differential effect of CCL2 on constitutive neutrophil apoptosis between normal and asthmatic subjects. J Cell Physiol 227(6):2567–2577. doi:10.1002/jcp.22995
Bolitho C, Hahn MA, Baxter RC, Marsh DJ (2010) The chemokine CXCL1 induces proliferation in epithelial ovarian cancer cells by transactivation of the epidermal growth factor receptor. Endocr Relat Cancer 17(4):929–940. doi:10.1677/ERC-10-0107
Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D et al (1995) The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem 270(45):27348–27357
Kuo PL, Shen KH, Hung SH, Hsu YL (2012) CXCL1/GROalpha increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-kappaB/HDAC1 epigenetic regulation. Carcinogenesis 33(12):2477–2487. doi:10.1093/carcin/bgs299
Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, Albini A, Lowell C, Berton G, Noonan DM, Cassatella MA (2004) CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol 172(8):5034–5040
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150(1):165–178
Nasser MW, Raghuwanshi SK, Grant DJ, Jala VR, Rajarathnam K, Richardson RM (2009) Differential activation and regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer. J Immunol 183(5):3425–3432. doi:10.4049/jimmunol.0900305
Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, Deryugina EI (2011) Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol 179(3):1455–1470. doi:10.1016/j.ajpath.2011.05.031
Dong C, Robertson GP (2009) Immunoediting of leukocyte functions within the tumor microenvironment promotes cancer metastasis development. Biorheology 46(4):265–279. doi:10.3233/BIR-2009-0545
Slattery MJ, Liang S, Dong C (2005) Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol 288(4):C831–C839. doi:10.1152/ajpcell.00439.2004
Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV (1998) Immune pathogenesis of hepatocellular carcinoma. J Exp Med 188(2):341–350
Rogler G (2013) Chronic ulcerative colitis and colorectal cancer. Cancer Lett. doi:10.1016/j.canlet.2013.07.032
Haqqani AS, Sandhu JK, Birnboim HC (2000) Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia 2(6):561–568
Knaapen AM, Gungor N, Schins RP, Borm PJ, Van Schooten FJ (2006) Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis 21(4):225–236. doi:10.1093/mutage/gel032
Güngör N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, Godschalk RW, van Schooten FJ (2010) Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 25(2):149–154. doi:10.1093/mutage/gep053
Gomez-Mejiba SE, Zhai Z, Gimenez MS, Ashby MT, Chilakapati J, Kitchin K, Mason RP, Ramirez DC (2010) Myeloperoxidase-induced genomic DNA-centered radicals. J Biol Chem 285(26):20062–20071. doi:10.1074/jbc.M109.086579
Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, Zhou XY, Zheng CL, Chi YY, Mukaida N, Li YY (2012) Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PLoS One 7(12):e51848. doi:10.1371/journal.pone.0051848
Ginzberg HH, Cherapanov V, Dong Q, Cantin A, McCulloch CA, Shannon PT, Downey GP (2001) Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol 281(3):G705–G717
Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hansch GM, Gaida MM (2012) Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol 2012:720768. doi:10.1155/2012/720768
Pekarek LA, Starr BA, Toledano AY, Schreiber H (1995) Inhibition of tumor growth by elimination of granulocytes. J Exp Med 181(1):435–440
Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M (2003) Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol 163(6):2221–2232. doi:10.1016/S0002-9440(10)63580-8
Coussens LM, Tinkle CL, Hanahan D, Werb Z (2000) MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103(3):481–490
Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C (2003) Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet 35(3):252–257. doi:10.1038/ng1249
Bodey B, Bodey B Jr, Siegel SE, Luck JV, Kaiser HE (1996) Immunophenotypic characterization of human primary and metastatic melanoma infiltrating leukocytes. Anticancer Res 16(6B):3439–3446
Yu J, Ren X, Chen Y, Liu P, Wei X, Li H, Ying G, Chen K, Winkler H, Hao X (2013) Dysfunctional activation of neurotensin/IL-8 pathway in hepatocellular carcinoma is associated with increased inflammatory response in microenvironment, more epithelial mesenchymal transition in cancer and worse prognosis in patients. PLoS One 8(2):e56069. doi:10.1371/journal.pone.0056069
Xie K (2001) Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev 12(4):375–391
Lippitz BE (2013) Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 14(6):e218–e228. doi:10.1016/S1470-2045(12)70582-X
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14(21):6735–6741. doi:10.1158/1078-0432.CCR-07-4843
Anderson IC, Mari SE, Broderick RJ, Mari BP, Shipp MA (2000) The angiogenic factor interleukin 8 is induced in non-small cell lung cancer/pulmonary fibroblast cocultures. Cancer Res 60(2):269–272
Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC (2003) Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res 9(2):729–737
Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE (1999) Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol 155(5):1557–1567. doi:10.1016/S0002-9440(10)65472-7
Yamano T, Morii E, Ikeda J, Aozasa K (2007) Granulocyte colony-stimulating factor production and rapid progression of gastric cancer after histological change in the tumor. Jpn J Clin Oncol 37(10):793–796. doi:10.1093/jjco/hym094
Shojaei F, Singh M, Thompson JD, Ferrara N (2008) Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A 105(7):2640–2645. doi:10.1073/pnas.0712185105
Schmielau J, Finn OJ (2001) Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res 61(12):4756–4760
Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M (2006) Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol 18(1):1–9. doi:10.1093/intimm/dxh334
Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M (2002) G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun 297(4):1058–1061
Voloshin T, Gingis-Velitski S, Bril R, Benayoun L, Munster M, Milsom C, Man S, Kerbel RS, Shaked Y (2011) G-CSF supplementation with chemotherapy can promote revascularization and subsequent tumor regrowth: prevention by a CXCR4 antagonist. Blood 118(12):3426–3435. doi:10.1182/blood-2010-11-320812
Shojaei F, Ferrara N (2008) Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res 68(14):5501–5504. doi:10.1158/0008-5472.CAN-08-0925
Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, Ho C, Ross J, Tan M, Carano RA, Meng YG, Ferrara N (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450(7171):825–831. doi:10.1038/nature06348
Qu X, Zhuang G, Yu L, Meng G, Ferrara N (2012) Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: key role of Stat3 signaling. J Biol Chem 287(23):19574–19584. doi:10.1074/jbc.M111.326801
Ohki Y, Heissig B, Sato Y, Akiyama H, Zhu Z, Hicklin DJ, Shimada K, Ogawa H, Daida H, Hattori K, Ohsaka A (2005) Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J 19(14):2005–2007. doi:10.1096/fj.04-3496fje
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744. doi:10.1038/35036374
Nozawa H, Chiu C, Hanahan D (2006) Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A 103(33):12493–12498. doi:10.1073/pnas.0601807103
Imai Y, Kubota Y, Yamamoto S, Tsuji K, Shimatani M, Shibatani N, Takamido S, Matsushita M, Okazaki K (2005) Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: an in vitro study. J Gastroenterol Hepatol 20(2):287–293. doi:10.1111/j.1440-1746.2004.03575.x
Di Carlo E, Forni G, Musiani P (2003) Neutrophils in the antitumoral immune response. Chem Immunol Allergy 83:182–203
Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P (2001) Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 189(2):197–206. doi:10.1002/jcp.10014
De Larco JE, Wuertz BR, Furcht LT (2004) The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 10(15):4895–4900. doi:10.1158/1078-0432.CCR-03-0760
Sun Z, Yang P (2004) Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol 5(3):182–190. doi:10.1016/S1470-2045(04)01414-7
Slattery MJ, Dong C (2003) Neutrophils influence melanoma adhesion and migration under flow conditions. Int J Cancer 106(5):713–722. doi:10.1002/ijc.11297
Huh SJ, Liang S, Sharma A, Dong C, Robertson GP (2010) Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res 70(14):6071–6082. doi:10.1158/0008-5472.CAN-09-4442
Drost EM, MacNee W (2002) Potential role of IL-8, platelet-activating factor and TNF-alpha in the sequestration of neutrophils in the lung: effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur J Immunol 32(2):393–403. doi:10.1002/1521-4141(200202)32:2<393::AID-IMMU393>3.0.CO;2–5
Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP (2001) Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol 280(4):C814–C822
Jadhav S, Bochner BS, Konstantopoulos K (2001) Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte-colon carcinoma cell adhesive interactions. J Immunol 167(10):5986–5993
Jadhav S, Konstantopoulos K (2002) Fluid shear- and time-dependent modulation of molecular interactions between PMNs and colon carcinomas. Am J Physiol Cell Physiol 283(4):C1133–C1143. doi:10.1152/ajpcell.00104.2002
Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M (2002) MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2(4):289–300
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827
Hiratsuka S, Watanabe A, Aburatani H, Maru Y (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8(12):1369–1375
Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L (2010) Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70(15):6139–6149. doi:10.1158/0008-5472.CAN-10-0706
Gao D, Joshi N, Choi H, Ryu S, Hahn M, Catena R, Sadik H, Argani P, Wagner P, Vahdat LT, Port JL, Stiles B, Sukumar S, Altorki NK, Rafii S, Mittal V (2012) Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res 72(6):1384–1394
Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, Sloan EK, Parker BS, Bowtell DD, Smyth MJ, Moller A (2012) Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 72(16):3906–3911. doi:10.1158/0008-5472.CAN-11-3873
Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ (2009) Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15(1):35–44. doi:10.1016/j.ccr.2008.11.012
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. doi:10.1172/JCI67484
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE (2012) Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72(16):3919–3927. doi:10.1158/0008-5472.CAN-11-2393
Bubenik J, Perlmann P, Helmstein K, Moberger G (1970) Cellular and humoral immune responses to human urinary bladder carcinomas. Int J Cancer 5(3):310–319
Shau H (1988) Cytostatic and tumoricidal activities of tumor necrosis factor-treated neutrophils. Immunol Lett 17(1):47–51
Shau H (1988) Characteristics and mechanism of neutrophil-mediated cytostasis induced by tumor necrosis factor. J Immunol 141(1):234–240
Miyake Y, Ajitsu S, Yamashita T, Sendo F (1988) Enhancement by recombinant interferon-gamma of spontaneous tumor cytostasis by human neutrophils. Mol Biother 1(1):37–42
Yamashita T, Uchida T, Araki A, Sendo F (1997) Nitric oxide is an effector molecule in inhibition of tumor cell growth by rIFN-gamma-activated rat neutrophils. Int J Cancer 71(2):223–230. doi:10.1002/(SICI)1097-0215(19970410)71:2<223::AID-IJC17>3.0.CO;2-I
Uchida T, Yamashita T, Araki A, Watanabe H, Sendo F (1997) rIFN-gamma-activated rat neutrophils induce tumor cell apoptosis by nitric oxide. Int J Cancer 71(2):231–236. doi:10.1002/(SICI)1097-0215(19970410)71:2<231::AID-IJC18>3.0.CO;2-K
Slivka A, LoBuglio AF, Weiss SJ (1980) A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood 55(2):347–350
Clark RA, Klebanoff SJ (1979) Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol 122(6):2605–2610
Weiss SJ, Slivka A (1982) Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J Clin Invest 69(2):255–262
Dallegri F, Frumento G, Patrone F (1983) Mechanisms of tumour cell destruction by PMA-activated human neutrophils. Immunology 48(2):273–279
Abrams SI, Brahmi Z (1984) Compared mechanisms of tumor cytolysis by human natural killer cells and activated polymorphonuclear leukocytes. J Immunol 132(6):3192–3196
Bru A, Albertos S, Lopez Garcia-Asenjo JA, Bru I (2004) Pinning of tumoral growth by enhancement of the immune response. Phys Rev Lett 92(23):238101
Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, Serio AM, Harrison LB, Bosl GJ, Pfister DG (2006) Double-blind, placebo-controlled, randomized trial of granulocyte-colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer J 12(3):182–188
Rini BI, Fong L, Weinberg V, Kavanaugh B, Small EJ (2006) Clinical and immunological characteristics of patients with serologic progression of prostate cancer achieving long-term disease control with granulocyte-macrophage colony-stimulating factor. J Urol 175(6):2087–2091. doi:10.1016/S0022-5347(06)00261-8
Bottoni U, Bonaccorsi P, Devirgiliis V, Panasiti V, Borroni RG, Trasimeni G, Clerico R, Calvieri S (2005) Complete remission of brain metastases in three patients with stage IV melanoma treated with BOLD and G-CSF. Jpn J Clin Oncol 35(9):507–513. doi:10.1093/jjco/hyi141
Borregaard N (2010) Neutrophils, from marrow to microbes. Immunity 33(5):657–670. doi:10.1016/j.immuni.2010.11.011
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3):159–175. doi:10.1038/nri3399
Dissemond J, Weimann TK, Schneider LA, Schneeberger A, Scharffetter-Kochanek K, Goos M, Wagner SN (2003) Activated neutrophils exert antitumor activity against human melanoma cells: reactive oxygen species-induced mechanisms and their modulation by granulocyte-macrophage-colony-stimulating factor. J Invest Dermatol 121(4):936–938. doi:10.1046/j.1523-1747.2003.12475.x
Zivkovic M, Poljak-Blazi M, Egger G, Sunjic SB, Schaur RJ, Zarkovic N (2005) Oxidative burst and anticancer activities of rat neutrophils. Biofactors 24(1–4):305–312
Clark RA, Klebanoff SJ (1975) Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med 141(6):1442–1447
Lichtenstein A, Kahle J (1985) Anti-tumor effect of inflammatory neutrophils: characteristics of in vivo generation and in vitro tumor cell lysis. Int J Cancer 35(1):121–127
Caruso RA, Speciale G, Inferrera C (1994) Neutrophil interaction with tumour cells in small early gastric cancer: ultrastructural observations. Histol Histopathol 9(2):295–303
Saito H, Fukumura D, Kurose I, Suematsu M, Tada S, Kagawa T, Miura S, Morizane T, Tsuchiya M (1992) Visualization of oxidative processes at the cellular level during neutrophil-mediated cytotoxicity against a human hepatoma cell line, HCC-M. Int J Cancer 51(1):124–129
Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P (2001) The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 97(2):339–345
Yamazaki M, Ikenami M, Sugiyama T (1988) Cytolytic heterogeneity of polymorphonuclear leukocytes in killing of murine tumor cells. Jpn J Cancer Res 79(11):1217–1223
Koga Y, Matsuzaki A, Suminoe A, Hattori H, Hara T (2004) Neutrophil-derived TNF-related apoptosis-inducing ligand (TRAIL): a novel mechanism of antitumor effect by neutrophils. Cancer Res 64(3):1037–1043
Al-Benna S, Shai Y, Jacobsen F, Steinstraesser L (2011) Oncolytic activities of host defense peptides. Int J Mol Sci 12(11):8027–8051. doi:10.3390/ijms12118027
Lichtenstein A, Ganz T, Selsted ME, Lehrer RI (1986) In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood 68(6):1407–1410
Kushner BH, Cheung NK (1992) Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood 79(6):1484–1490
Iliopoulos D, Ernst C, Steplewski Z, Jambrosic JA, Rodeck U, Herlyn M, Clark WH Jr, Koprowski H, Herlyn D (1989) Inhibition of metastases of a human melanoma xenograft by monoclonal antibody to the GD2/GD3 gangliosides. J Natl Cancer Inst 81(6):440–444
Valerius T, Repp R, de Wit TP, Berthold S, Platzer E, Kalden JR, Gramatzki M, van de Winkel JG (1993) Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood 82(3):931–939
Lieschke GJ, Burgess AW (1992) Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2). N Engl J Med 327(2):99–106. doi:10.1056/NEJM199207093270207
Clynes RA, Towers TL, Presta LG, Ravetch JV (2000) Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 6(4):443–446. doi:10.1038/74704
Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS (2003) Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin’s lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res 9(16 Pt 1):5866–5873
Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S (2011) Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res 71(15):5134–5143. doi:10.1158/0008-5472.CAN-10-4222
Guettinger Y, Barbin K, Peipp M, Bruenke J, Dechant M, Horner H, Thierschmidt D, Valerius T, Repp R, Fey GH, Stockmeyer B (2010) A recombinant bispecific single-chain fragment variable specific for HLA class II and Fc alpha RI (CD89) recruits polymorphonuclear neutrophils for efficient lysis of malignant B lymphoid cells. J Immunol 184(3):1210–1217. doi:10.4049/jimmunol.0902033
Stockmeyer B, Dechant M, van Egmond M, Tutt AL, Sundarapandiyan K, Graziano RF, Repp R, Kalden JR, Gramatzki M, Glennie MJ, van de Winkel JG, Valerius T (2000) Triggering Fc alpha-receptor I (CD89) recruits neutrophils as effector cells for CD20-directed antibody therapy. J Immunol 165(10):5954–5961
Otten MA, Rudolph E, Dechant M, Tuk CW, Reijmers RM, Beelen RH, van de Winkel JG, van Egmond M (2005) Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol 174(9):5472–5480
Cheung IY, Hsu K, Cheung NK (2012) Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol 30(4):426–432. doi:10.1200/JCO.2011.37.6236
Mocsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210(7):1283–1299. doi:10.1084/jem.20122220
Tanaka E, Sendo F (1993) Abrogation of tumor-inhibitory MRC-OX8+ (CD8+) effector T-cell generation in rats by selective depletion of neutrophils in vivo using a monoclonal antibody. Int J Cancer 54(1):131–136
Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R, Zychlinsky A (2013) A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 4:48. doi:10.3389/fimmu.2013.00048
Tillack K, Breiden P, Martin R, Sospedra M (2012) T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 188(7):3150–3159. doi:10.4049/jimmunol.1103414
Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ (2008) Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol 20(11):1467–1479. doi:10.1093/intimm/dxn104
Takami K, Miura K, Takeuchi H, Egawa S, Moriya T, Nakamura Y, Tanabe A, Sugita J, Karasawa H, Unno M, Sasaki I (2008) Granulocyte-colony stimulating factor-producing pancreatic cancer: report of a case. Surg Today 38(5):453–457. doi:10.1007/s00595-007-3636-z
Fujiwara Y, Yamazaki O, Takatsuka S, Kaizaki R, Inoue T (2011) Granulocyte colony-stimulating factor-producing ascending colon cancer as indicated by histopathological findings: report of a case. Osaka City Med J 57(2):79–84
Kaira K, Ishizuka T, Tanaka H, Tanaka Y, Yanagitani N, Sunaga N, Hisada T, Mori M (2008) Lung cancer producing granulocyte colony-stimulating factor and rapid spreading to peritoneal cavity. J Thorac Oncol 3(9):1054–1055. doi:10.1097/JTO.0b013e3181834f7b
Matsuyama S, Shimonishi T, Yoshimura H, Higaki K, Nasu K, Toyooka M, Aoki S, Watanabe K, Sugihara H (2008) An autopsy case of granulocyte-colony-stimulating-factor-producing extrahepatic bile duct carcinoma. World J Gastroenterol 14(18):2924–2927
Kawaguchi M, Asada Y, Terada T, Takehara A, Munemoto Y, Fujisawa K, Mitsui T, Iida Y, Miura S, Sudo Y (2010) Aggressive recurrence of gastric cancer as a granulocyte-colony-stimulating factor-producing tumor. Int J Clin Oncol 15(2):191–195. doi:10.1007/s10147-010-0023-3
Ito S, Iwai Y, Fujii T, Yoshida N, Hayashi S (1999) Two cases of bladder tumor producing granulocyte colony stimulating factor. Hinyokika Kiyo 45(1):57–60
Nasu K, Inoue C, Takai N, Kashima K, Miyakawa I (2004) Squamous cell carcinoma of the cervix producing granulocyte colony-stimulating factor. Obstet Gynecol 104(5 Pt 1):1086–1088. doi:10.1097/01.AOG.0000141552.87313.c8
Horii A, Shimamura K, Honjo Y, Mitani K, Miki T, Takashima S, Yoshida J (1997) Granulocyte colony stimulating factor-producing tongue carcinoma. Head Neck 19(4):351–356. doi:10.1002/(SICI)1097-0347(199707)19:4<351::AID-HED15>3.0.CO;2-C
Sakamoto A, Yamamoto H, Tanaka K, Matsuda S, Harimaya K, Oda Y, Tsuneyoshi M, Iwamoto Y (2006) Dedifferentiated chondrosarcoma with leukocytosis and elevation of serum G-CSF. A case report. World J Surg Oncol 4:37
Sakamoto A, Matono H, Yoshida T, Tanaka K, Matsuda S, Oda Y, Iwamoto Y (2007) Dedifferentiated liposarcoma with leukocytosis. A case report of G-CSF-producing soft-tissue tumors, possible association with undifferentiated liposarcoma lineage. World J Surg Oncol 5:131
Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, Iezzi G, Sconocchia G, Heberer M, Lugli A, Tornillo L, Oertli D, Terracciano L, Spagnoli GC (2013) High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One 8(5):e64814. doi:10.1371/journal.pone.0064814
Hirt C, Eppenberger-Castori S, Sconocchia G, Iezzi G, Tornillo L, Terracciano L, Spagnoli GC, Droeser RA (2013) Colorectal carcinoma infiltration by myeloperoxidase-expressing neutrophil granulocytes is associated with favorable prognosis. Oncoimmunology 2(10):e25990. doi:10.4161/onci.25990
Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, Zuber M, Droeser R, Muraro MG, Mengus C, Oertli D, Ferrone S, Terracciano L, Spagnoli GC (2011) Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer 128(11):2663–2672. doi:10.1002/ijc.25609
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, Cai MY, Xie D (2012) Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS One 7(1):e30806. doi:10.1371/journal.pone.0030806
Riesco A (1970) Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer 25(1):135–140
Donskov F (2013) Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 23(3):200–207. doi:10.1016/j.semcancer.2013.02.001
Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, Tsuboi M, Shibata K, Furuse K, Fukushima M (2009) Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer 45(11):1950–1958. doi:10.1016/j.ejca.2009.01.023
Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S (2010) Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 102(7):742–747. doi:10.1002/jso.21725
Donskov F, von der Maase H (2006) Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol 24(13):1997–2005. doi:10.1200/JCO.2005.03.9594
Wang GY, Yang Y, Zhang Q, Li H, Chen GZ, Yi SH, Xu C, Wang GS, Zhang J, Yi HM, Jiang N, Fu BS, Zhao H, Li MR, Chen YH, Cai CJ, Lu MQ, Chen GH (2011) Preoperative neutrophil-lymphocyte ratio as a prognostic predictor after liver transplantation for hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 91(22):1519–1522
Hung HY, Chen JS, Yeh CY, Changchien CR, Tang R, Hsieh PS, Tasi WS, You JF, You YT, Fan CW, Wang JY, Chiang JM (2011) Effect of preoperative neutrophil-lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int J Colorectal Dis 26(8):1059–1065. doi:10.1007/s00384-011-1192-x
Liu H, Ubukata H, Tabuchi T, Takemura A, Motohashi G, Nishimura M, Satani T, Hong J, Katano M, Nakada I, Saniabadi AR (2009) It is possible that tumour-infiltrating granulocytes promote tumour progression. Oncol Rep 22(1):29–33
Trellakis S, Farjah H, Bruderek K, Dumitru CA, Hoffmann TK, Lang S, Brandau S (2011) Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int J Immunopathol Pharmacol 24(3):683–693
Dumitru CA, Fechner MK, Hoffmann TK, Lang S, Brandau S (2012) A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J Leukoc Biol 91(4):591–598. doi:10.1189/jlb.0411193
Dumitru CA, Gholaman H, Trellakis S, Bruderek K, Dominas N, Gu X, Bankfalvi A, Whiteside TL, Lang S, Brandau S (2011) Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer 129(4):859–869. doi:10.1002/ijc.25991
Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S (2008) Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett 261(2):147–157. doi:10.1016/j.canlet.2007.11.028
Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen Y, Jiang G, Lau C, Yu WC, Bacher M, Fan ST (2003) Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int J Cancer 107(1):22–29. doi:10.1002/ijc.11287
Ren Y, Law S, Huang X, Lee PY, Bacher M, Srivastava G, Wong J (2005) Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann Surg 242(1):55–63
Rendon BE, Willer SS, Zundel W, Mitchell RA (2009) Mechanisms of macrophage migration inhibitory factor (MIF)-dependent tumor microenvironmental adaptation. Exp Mol Pathol 86(3):180–185. doi:10.1016/j.yexmp.2009.01.001
Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, Huang XW, Fan J, Zhou J (2012) Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 56(6):2242–2254. doi:10.1002/hep.25907
Ietomi K (1990) A study on the role of granulocytes in carcinoma-bearing hosts–G/L ratio as a new host indicator. Nihon Gan Chiryo Gakkai Shi 25(3):662–671
Terasawa M, Nagata K, Kobayashi Y (2008) Neutrophils and monocytes transport tumor cell antigens from the peritoneal cavity to secondary lymphoid tissues. Biochem Biophys Res Commun 377(2):589–594. doi:10.1016/j.bbrc.2008.10.011
Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI (2004) Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol 172(2):989–999
Patel KD, Zimmerman GA, Prescott SM, McEver RP, McIntyre TM (1991) Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol 112(4):749–759
Cherdyntseva NV, Bogdashin IV, Nebera SA, Vasil’ev NV (1989) Neutrophil granulocyte function in patients with malignant neoplasms. Vopr Onkol 35(4):429–433
Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF (2011) Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 54(3):497–505. doi:10.1016/j.jhep.2010.07.044
Tsuda Y, Fukui H, Asai A, Fukunishi S, Miyaji K, Fujiwara S, Teramura K, Fukuda A, Higuchi K (2012) An immunosuppressive subtype of neutrophils identified in patients with hepatocellular carcinoma. J Clin Biochem Nutr 51(3):204–212. doi:10.3164/jcbn.12-32
Jensen TO, Schmidt H, Moller HJ, Donskov F, Hoyer M, Sjoegren P, Christensen IJ, Steiniche T (2012) Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 118(9):2476–2485. doi:10.1002/cncr.26511
Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H (2009) Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 27(28):4709–4717. doi:10.1200/JCO.2008.18.9498
Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM (2011) Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 225(3):438–447. doi:10.1002/path.2947
Chee DO, Townsend CM Jr, Galbraith MA, Eilber FR, Morton DL (1978) Selective reduction of human tumor cell populations by human granulocytes in vitro. Cancer Res 38(12):4534–4539
Halbrecht I, Komlos L (1974) Cytotoxic effects of leukocytes and plasma on primary cultures of ovarian carcinoma. Obstet Gynecol 43(2):268–275
Korec S, Herberman RB, Dean JH, Cannon GB (1980) Cytostasis of tumor cell lines by human granulocytes. Cell Immunol 53(1):104–115
Gerrard TL, Cohen DJ, Kaplan AM (1981) Human neutrophil-mediated cytotoxicity to tumor cells. J Natl Cancer Inst 66(3):483–488
Cameron DJ (1983) A comparison of the cytotoxic potential in polymorphonuclear leukocytes obtained from normal donors and cancer patients. Clin Immunol Immunopathol 28(1):115–124
Cameron DJ (1986) Lidocaine inhibits macrophage mediated cytotoxicity and enhances neutrophil mediated cytotoxicity. Jpn J Exp Med 56(6):265–269
Cameron DJ (1985) Inhibition of neutrophil-mediated cytotoxicity by exogenous adenosine 5′-triphosphate. Clin Immunol Immunopathol 37(2):230–235
Cameron DJ, Majeski JA (1988) Inhibition of macrophage- and neutrophil-mediated cytotoxicity by verapamil. J Surg Oncol 37(1):5–9
Cameron DJ (1986) Role of hydrocortisone, chloroquine and prednisolone in neutrophil mediated cytotoxicity. Jpn J Exp Med 56(5):207–212
Sauri H, Kim AT, Shau H (1995) Characterization of an oxidation-resistant tumor cell line and its sensitivity to immune response and chemotherapy. J Surg Res 58(5):526–535. doi:10.1006/jsre.1995.1083
Heidecke H, Eckert K, Schulze-Forster K, Maurer HR (1997) Prothymosin alpha 1 effects in vitro on chemotaxis, cytotoxicity and oxidative response of neutrophils from melanoma, colorectal and breast tumor patients. Int J Immunopharmacol 19(8):413–420
Godleski JJ, Lee RE, Leighton J (1970) Studies on the role of polymorphonuclear leukocytes in neoplastic disease with the chick embryo and Walker carcinosarcoma 256 in vivo and in vitro. Cancer Res 30(7):1986–1993
Pickaver AH, Ratcliffe NA, Williams AE, Smith H (1972) Cytotoxic effects of peritoneal neutrophils on a syngeneic rat tumour. Nat New Biol 235(58):186–187
Fisher B, Saffer EA (1978) Tumor cells cytotoxicity by granulocytes from peripheral blood of tumor-bearing mice. J Natl Cancer Inst 60(3):687–691
Nathan CF, Brukner LH, Silverstein SC, Cohn ZA (1979) Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med 149(1):84–99
Becker S, Haskill S (1980) Non-T-cell-mediated cytotoxicity in mice with tumors induced by Moloney murine sarcoma virus (M-MuSV). II. Granulocyte-mediatd cytotoxicity against autochthonous target cells isolated from M-MuSV-induced tumors. J Natl Cancer Inst 65(2):469–475
Morikawa K, Kamegaya S, Yamazaki M, Mizuno D (1985) Hydrogen peroxide as a tumoricidal mediator of murine polymorphonuclear leukocytes induced by a linear beta-1,3-D-glucan and some other immunomodulators. Cancer Res 45(8):3482–3486
Morikawa K, Takeda R, Yamazaki M, Mizuno D (1985) Induction of tumoricidal activity of polymorphonuclear leukocytes by a linear beta-1,3-D-glucan and other immunomodulators in murine cells. Cancer Res 45(4):1496–1501
Lichtenstein AK, Kahle J, Berek J, Zighelboim J (1984) Successful immunotherapy with intraperitoneal Corynebacterium parvum in a murine ovarian cancer model is associated with the recruitment of tumor-lytic neutrophils into the peritoneal cavity. J Immunol 133(1):519–526
Hirose K, Hakozaki M, Nyunoya Y, Kobayashi Y, Matsushita K, Takenouchi T, Mikata A, Mukaida N, Matsushima K (1995) Chemokine gene transfection into tumour cells reduced tumorigenicity in nude mice in association with neutrophilic infiltration. Br J Cancer 72(3):708–714
Chen RL, Reynolds CP, Seeger RC (2000) Neutrophils are cytotoxic and growth-inhibiting for neuroblastoma cells with an anti-GD2 antibody but, without cytotoxicity, can be growth-stimulating. Cancer Immunol Immunother 48(11):603–612
Lozupone F, Luciani F, Venditti M, Rivoltini L, Pupa S, Parmiani G, Belardelli F, Fais S (2000) Murine granulocytes control human tumor growth in SCID mice. Int J Cancer 87(4):569–573
Jaganjac M, Poljak-Blazi M, Zarkovic K, Schaur RJ, Zarkovic N (2008) The involvement of granulocytes in spontaneous regression of Walker 256 carcinoma. Cancer Lett 260(1–2):180–186. doi:10.1016/j.canlet.2007.10.039
Jaganjac M, Poljak-Blazi M, Kirac I, Borovic S, Joerg Schaur R, Zarkovic N (2010) Granulocytes as effective anticancer agent in experimental solid tumor models. Immunobiology 215(12):1015–1020. doi:10.1016/j.imbio.2010.01.002
Jaganjac M, Poljak-Blazi M, Schaur RJ, Zarkovic K, Borovic S, Cipak A, Cindric M, Uchida K, Waeg G, Zarkovic N (2012) Elevated neutrophil elastase and acrolein-protein adducts are associated with W256 regression. Clin Exp Immunol 170(2):178–185. doi:10.1111/j.1365-2249.2012.04639.x
Ibata M, Takahashi T, Shimizu T, Inoue Y, Maeda S, Tashiro-Yamaji J, Okada M, Ueda K, Kubota T, Yoshida R (2011) Spontaneous rejection of intradermally transplanted non-engineered tumor cells by neutrophils and macrophages from syngeneic strains of mice. Microbiol Immunol 55(10):726–735. doi:10.1111/j.1348-0421.2011.00369.x
Edelson PJ, Cohn ZA (1973) Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med 138(1):318–323
Clark RA, Klebanoff SJ, Einstein AB, Fefer A (1975) Peroxidase-H2O2-halide system: cytotoxic effect on mammalian tumor cells. Blood 45(2):161–170
Clark RA, Olsson I, Klebanoff SJ (1976) Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol 70(3):719–723
Ikenami M, Yamazaki M (1985) Participation of polymorphonuclear leukocyte-derived factor in murine tumour cell killing. Br J Cancer 52(4):575–581
Mikami M, Yamazaki M, Yui S (1998) Kinetical analysis of tumor cell death-inducing mechanism by polymorphonuclear leukocyte-derived calprotectin: involvement of protein synthesis and generation of reactive oxygen species in target cells. Microbiol Immunol 42(3):211–221
Noh H, Eomm M, Han A (2013) Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer 16(1):55–59. doi:10.4048/jbc.2013.16.1.55
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, Widmann WD (2012) Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 19(1):217–224. doi:10.1245/s10434-011-1814-0
Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, Jung HS, Lee S (2013) Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer 13:78. doi:10.1186/1471-2407-13-78
Tibaldi C, Vasile E, Bernardini I, Orlandini C, Andreuccetti M, Falcone A (2008) Baseline elevated leukocyte count in peripheral blood is associated with poor survival in patients with advanced non-small cell lung cancer: a prognostic model. J Cancer Res Clin Oncol 134(10):1143–1149. doi:10.1007/s00432-008-0378-2
Satomi A, Murakami S, Ishida K, Mastuki M, Hashimoto T, Sonoda M (1995) Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol 34(1):69–73
Shibutani M, Maeda K, Nagahara H, Noda E, Ohtani H, Nishiguchi Y, Hirakawa K (2013) A high preoperative neutrophil-to-lymphocyte ratio is associated with poor survival in patients with colorectal cancer. Anticancer Res 33(8):3291–3294
Chua W, Charles KA, Baracos VE, Clarke SJ (2011) Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104(8):1288–1295. doi:10.1038/bjc.2011.100
He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, Chen X, Rong R, Zhang B, Xia L (2013) Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol 30(1):439. doi:10.1007/s12032-012-0439-x
Ohno Y, Nakashima J, Ohori M, Hatano T, Tachibana M (2010) Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of recurrence in patients with nonmetastatic renal cell carcinoma. J Urol 184(3):873–878. doi:10.1016/j.juro.2010.05.028
Atzpodien J, Royston P, Wandert T, Reitz M (2003) Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer 88(3):348–353. doi:10.1038/sj.bjc.6600768
Can C, Baseskioglu B, Yilmaz M, Colak E, Ozen A, Yenilmez A (2012) Pretreatment parameters obtained from peripheral blood sample predicts invasiveness of bladder carcinoma. Urol Int 89(4):468–472. doi:10.1159/000343278
Perisanidis C, Kornek G, Poschl PW, Holzinger D, Pirklbauer K, Schopper C, Ewers R (2013) High neutrophil-to-lymphocyte ratio is an independent marker of poor disease-specific survival in patients with oral cancer. Med Oncol 30(1):334. doi:10.1007/s12032-012-0334-5
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18(12):3362–3369. doi:10.1245/s10434-011-1754-8
Sato H, Tsubosa Y, Kawano T (2012) Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg 36(3):617–622. doi:10.1007/s00268-011-1411-1
Rashid F, Waraich N, Bhatti I, Saha S, Khan RN, Ahmed J, Leeder PC, Larvin M, Iftikhar SY (2010) A pre-operative elevated neutrophil: lymphocyte ratio does not predict survival from oesophageal cancer resection. World J Surg Oncol 8:1. doi:10.1186/1477-7819-8-1
Wang L, Lin Y, Long H, Liu H, Rao H, He Y, Rong T, Liang Y (2013) Esophageal carcinosarcoma: a unique entity with better prognosis. Ann Surg Oncol 20(3):997–1004. doi:10.1245/s10434-012-2658-y
Kim YH, Choi WJ (2012) The effectiveness of postoperative neutrophils to lymphocytes ratio in predicting long-term recurrence after stomach cancer surgery. J Korean Surg Soc 83(6):352–359. doi:10.4174/jkss.2012.83.6.352
Kao SC, Pavlakis N, Harvie R, Vardy JL, Boyer MJ, van Zandwijk N, Clarke SJ (2010) High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 16(23):5805–5813. doi:10.1158/1078-0432.CCR-10-2245
Kao SC, Klebe S, Henderson DW, Reid G, Chatfield M, Armstrong NJ, Yan TD, Vardy J, Clarke S, van Zandwijk N, McCaughan B (2011) Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 6(11):1923–1929. doi:10.1097/JTO.0b013e31822a3740
Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R (2012) Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol 7(3):587–594. doi:10.1097/JTO.0b013e31823f45c1
Wang S, Zhang Z, Fang F, Gao X, Sun W, Liu H (2011) The neutrophil/lymphocyte ratio is an independent prognostic indicator in patients with bone metastasis. Oncol Lett 2(4):735–740. doi:10.3892/ol.2011.304
Acknowledgments
We thank Dr. Seth Salpeter for critical reading of this review. Research in the authors’ laboratory is supported by the I-CORE Program of The Israel Science Foundation (Grant No. 41/11), The Abisch-Frenkel Foundation and The Rosetrees Trust (R.V.S and Z.G).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sionov, R.V., Fridlender, Z.G. & Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenvironment 8, 125–158 (2015). https://doi.org/10.1007/s12307-014-0147-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-014-0147-5