Abstract
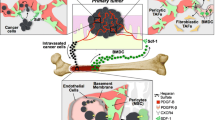
Tumor growth relies on the formation of new blood vessels to receive an adequate supply of oxygen and nutrient. This process is facilitated by both the remodeling of the pre-existing vasculatures and the recruitment of the progenitor/stem cells originated from bone marrow-derived cells (BMDCs). Evidences from both animal studies and human trials have reported that these tumor-associated BMDCs differentiate into a series of stromal cells including macrophages and pericytes, and regulate tumor angiogenesis in various aspects. Macrophages constitute a large portion of the BMDCs infiltrated in the tumor microenvironment, and have been shown to disrupt the balance of pro- and anti-angiogenic signalings by the secretion of various cytokines. Pericytes, mainly derived from the subpopulation of PDGFRβ+ BMDCs, can provide both pro-survival signaling and mechanical support to maintain the newly formed endothelium via the direct interactions with endothelial cells. In the current review, we summarize the recruitment mechanisms of BMDC-derived macrophages and pericytes within tumor microenvironment, and also review the contribution of these cells to the different aspects of angiogenesis, with particular emphasis on their therapeutic implications as potential targets for anti-tumor strategies.
Similar content being viewed by others
References
Joyce JA (2005) Therapeutic targeting of the tumor microenvironment. Cancer Cell 7(6):513–520. doi:10.1016/j.ccr.2005.05.024
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86(3):353–364
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Ding YT, Kumar S, Yu DC (2008) The role of endothelial progenitor cells in tumour vasculogenesis. Pathobiology 75(5):265–273. doi:10.1159/000151706
Nyberg P, Salo T, Kalluri R (2008) Tumor microenvironment and angiogenesis. Front Biosci 13:6537–6553
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8(8):618–631. doi:10.1038/nrc2444
Solinas G, Germano G, Mantovani A, Allavena P (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073. doi:10.1189/jlb.0609385
Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, Fu Y, Luo Y (2009) Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res 69(15):6057–6064. doi:10.1158/0008-5472.CAN-08-2007
Murdoch C, Giannoudis A, Lewis CE (2004) Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 104(8):2224–2234. doi:10.1182/blood-2004-03-11092004-03-1109
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12(2):121–127
Murdoch C, Finn A (2000) Chemokine receptors and their role in inflammation and infectious diseases. Blood 95(10):3032–3043
Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A (1983) Regulation of the macrophage content of neoplasms by chemoattractants. Science 220(4593):210–212
Han KH, Tangirala RK, Green SR, Quehenberger O (1998) Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol 18(12):1983–1991
Graves DT, Barnhill R, Galanopoulos T, Antoniades HN (1992) Expression of monocyte chemotactic protein-1 in human melanoma in vivo. Am J Pathol 140(1):9–14
Negus RP, Stamp GW, Relf MG, Burke F, Malik ST, Bernasconi S, Allavena P, Sozzani S, Mantovani A, Balkwill FR (1995) The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest 95(5):2391–2396. doi:10.1172/JCI117933
Mazzucchelli L, Loetscher P, Kappeler A, Uguccioni M, Baggiolini M, Laissue JA, Mueller C (1996) Monocyte chemoattractant protein-1 gene expression in prostatic hyperplasia and prostate adenocarcinoma. Am J Pathol 149(2):501–509
Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST (1997) Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol 93(5):518–527
Miotto D, Boschetto P, Bononi I, Milani G, Legorini C, Cavallesco G, Lo Cascio N, Zeni E, Fabbri LM, Mapp CE (2007) CC ligand 2 levels are increased in LPS-stimulated peripheral monocytes of patients with non-small cell lung cancer. Respir Med 101(8):1738–1743. doi:10.1016/j.rmed.2007.02.021
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6(8):3282–3289
Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S (2004) Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol 99(9):1667–1674. doi:10.1111/j.1572-0241.2004.30733.x
Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K (2003) Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol 22(4):773–778
Bottazzi B, Walter S, Govoni D, Colotta F, Mantovani A (1992) Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J Immunol 148(4):1280–1285
Nesbit M, Schaider H, Miller TH, Herlyn M (2001) Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 166(11):6483–6490
Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, Chayama K (2005) Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res 11(21):7629–7636. doi:10.1158/1078-0432.CCR-05-0798
Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A (2009) Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer 125(6):1276–1284. doi:10.1002/ijc.24378
Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ (2009) The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia 11(11):1235–1242
Scotton C, Milliken D, Wilson J, Raju S, Balkwill F (2001) Analysis of CC chemokine and chemokine receptor expression in solid ovarian tumours. Br J Cancer 85(6):891–897. doi:10.1054/bjoc.2001.2020S0007092001920208
Tseng D, Vasquez-Medrano DA, Brown JM (2011) Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer 104 (12):1805–1809. doi:10.1038/bjc.2011.169
Locati M, Deuschle U, Massardi ML, Martinez FO, Sironi M, Sozzani S, Bartfai T, Mantovani A (2002) Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J Immunol 168(7):3557–3562
Robinson SC, Scott KA, Balkwill FR (2002) Chemokine stimulation of monocyte matrix metalloproteinase-9 requires endogenous TNF-alpha. Eur J Immunol 32(2):404–412. doi:10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X
Schmid MC, Varner JA (2007) Myeloid cell trafficking and tumor angiogenesis. Cancer Lett 250(1):1–8. doi:10.1016/j.canlet.2006.09.002
Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D (1996) Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87(8):3336–3343
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68(11):4340–4346. doi:10.1158/0008-5472.CAN-07-6705
Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM (2012) Vascular endothelial growth factor induced skin carcinogenesis depends on recruitment and alternative activation of macrophages. J Pathol. doi:10.1002/path.3989
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M, De Mol M, Autiero M, Wyns S, Plaisance S, Moons L, van Rooijen N, Giacca M, Stassen JM, Dewerchin M, Collen D, Carmeliet P (2007) Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131(3):463–475. doi:10.1016/j.cell.2007.08.038
Ding Y, Huang Y, Song N, Gao X, Yuan S, Wang X, Cai H, Fu Y, Luo Y (2010) NFAT1 mediates placental growth factor-induced myelomonocytic cell recruitment via the induction of TNF-alpha. J Immunol 184(5):2593–2601. doi:10.4049/jimmunol.0902378
Hiratsuka S, Watanabe A, Aburatani H, Maru Y (2006) Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8(12):1369–1375. doi:10.1038/ncb1507
Allavena P, Sica A, Garlanda C, Mantovani A (2008) The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 222:155–161. doi:10.1111/j.1600-065X.2008.00607.x
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686. doi:10.1016/j.it.2004.09.015
Mantovani A, Sica A, Locati M (2005) Macrophage polarization comes of age. Immunity 23(4):344–346. doi:10.1016/j.immuni.2005.10.001
Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A (2008) Macrophage polarization in tumour progression. Semin Cancer Biol 18(5):349–355. doi:10.1016/j.semcancer.2008.03.004
Biswas SK, Sica A, Lewis CE (2008) Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 180(4):2011–2017
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141(1):39–51. doi:10.1016/j.cell.2010.03.014
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Dinapoli MR, Calderon CL, Lopez DM (1996) The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med 183(4):1323–1329
Klimp AH, Hollema H, Kempinga C, van der Zee AG, de Vries EG, Daemen T (2001) Expression of cyclooxygenase-2 and inducible nitric oxide synthase in human ovarian tumors and tumor-associated macrophages. Cancer Res 61(19):7305–7309
Ruffell B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol. doi:10.1016/j.it.2011.12.001
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10(8):858–864. doi:10.1038/nm1075
Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC (2010) Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120(8):2699–2714. doi:10.1172/JCI39506
Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70(19):7465–7475. doi:10.1158/0008-5472.CAN-10-1439
Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A (2000) Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol 164(2):762–767
Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ (2002) Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol 169(5):2253–2263
Allavena P, Sica A, Vecchi A, Locati M, Sozzani S, Mantovani A (2000) The chemokine receptor switch paradigm and dendritic cell migration: its significance in tumor tissues. Immunol Rev 177:141–149
Kambayashi T, Alexander HR, Fong M, Strassmann G (1995) Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol 154(7):3383–3390
Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A (2009) The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30(3):377–386. doi:10.1093/carcin/bgp014
Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376 (9754):1741–1750. doi:10.1016/S0140-6736(10)61543-7
Nakanishi Y, Nakatsuji M, Seno H, Ishizu S, Akitake-Kawano R, Kanda K, Ueo T, Komekado H, Kawada M, Minami M, Chiba T (2011) COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 32(9):1333–1339. doi:10.1093/carcin/bgr128
Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, Song X, Luo Y (2011) Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res 17(23):7230–7239. doi:10.1158/1078-0432.CCR-11-1354
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL (1996) Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res 56(20):4625–4629
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17(3):445–451
Fujimoto J, Sakaguchi H, Aoki I, Tamaya T (2000) Clinical implications of expression of interleukin 8 related to angiogenesis in uterine cervical cancers. Cancer Res 60(10):2632–2635
Hashimoto I, Kodama J, Seki N, Hongo A, Miyagi Y, Yoshinouchi M, Kudo T (2000) Macrophage infiltration and angiogenesis in endometrial cancer. Anticancer Res 20(6C):4853–4856
Peng SH, Deng H, Yang JF, Xie PP, Li C, Li H, Feng DY (2005) Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol 11(41):6521–6524
Hanada T, Nakagawa M, Emoto A, Nomura T, Nasu N, Nomura Y (2000) Prognostic value of tumor-associated macrophage count in human bladder cancer. Int J Urol 7(7):263–269
Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, Yamada T, Itoyama S, Ito H, Okada K (2002) Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res 22(6C):4281–4284
Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M (1999) Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 5(5):1107–1113
Makitie T, Summanen P, Tarkkanen A, Kivela T (2001) Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 42(7):1414–1421
Li C, Shintani S, Terakado N, Nakashiro K, Hamakawa H (2002) Infiltration of tumor-associated macrophages in human oral squamous cell carcinoma. Oncol Rep 9(6):1219–1223
Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, Yamada H, Okada S, Watari K, Ono M, Kuwano M, Kamura S, Iida K, Okada Y, Koga M, Iwamoto Y (2011) Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol 179(3):1157–1170. doi:10.1016/j.ajpath.2011.05.034
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612. doi:10.1158/0008-5472.CAN-05-4005
Aharinejad S, Paulus P, Sioud M, Hofmann M, Zins K, Schafer R, Stanley ER, Abraham D (2004) Colony-stimulating factor-1 blockade by antisense oligonucleotides and small interfering RNAs suppresses growth of human mammary tumor xenografts in mice. Cancer Res 64(15):5378–5384. doi:10.1158/0008-5472.CAN-04-096164/15/5378
Lin EY, Pollard JW (2007) Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res 67(11):5064–5066. doi:10.1158/0008-5472.CAN-07-0912
Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ (2006) Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer 94(1):101–107. doi:10.1038/sj.bjc.6602901
Kimura YN, Watari K, Fotovati A, Hosoi F, Yasumoto K, Izumi H, Kohno K, Umezawa K, Iguchi H, Shirouzu K, Takamori S, Kuwano M, Ono M (2007) Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci 98(12):2009–2018. doi:10.1111/j.1349-7006.2007.00633.x
Bicknell R, Harris AL (1991) Novel growth regulatory factors and tumour angiogenesis. Eur J Cancer 27(6):781–785
Li C, Liu B, Dai Z, Tao Y (2011) Knockdown of VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration, angiogenesis and growth of clear cell renal cell carcinoma (CRCC). Cancer Biol Ther 12(10):872–880. doi:10.4161/cbt.12.10.17672
Zhang W, Wang L, Zhou D, Cui Q, Zhao D, Wu Y (2011) Expression of tumor-associated macrophages and vascular endothelial growth factor correlates with poor prognosis of peripheral T-cell lymphoma, not otherwise specified. Leuk Lymphoma 52(1):46–52. doi:10.3109/10428194.2010.529204
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S (2005) Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep 14(2):425–431
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676. doi:10.1038/nm0603-669
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. doi:10.1016/S0140-6736(00)04046-0
Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A (2003) Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198(9):1391–1402. doi:10.1084/jem.20030267
Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, Li G, Lu X, Strieter RM, Burdick M, Go VL, Reber HA, Eibl G, Hines OJ (2011) Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol 178 (3):1340–1349. doi:10.1016/j.ajpath.2010.11.058
Strieter RM, Belperio JA, Phillips RJ, Keane MP (2004) CXC chemokines in angiogenesis of cancer. Semin Cancer Biol 14(3):195–200. doi:10.1016/j.semcancer.2003.10.006
Rosenbaum JT, Howes EL Jr, Rubin RM, Samples JR (1988) Ocular inflammatory effects of intravitreally-injected tumor necrosis factor. Am J Pathol 133(1):47–53
Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N (1987) Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329(6140):630–632. doi:10.1038/329630a0
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258(5089):1798–1801
Motro B, Itin A, Sachs L, Keshet E (1990) Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A 87(8):3092–3096
West DC, Hampson IN, Arnold F, Kumar S (1985) Angiogenesis induced by degradation products of hyaluronic acid. Science 228(4705):1324–1326
Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y (2006) Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells 24(12):2733–2743. doi:10.1634/stemcells.2006-0026
Koga M, Kai H, Egami K, Murohara T, Ikeda A, Yasuoka S, Egashira K, Matsuishi T, Kai M, Kataoka Y, Kuwano M, Imaizumi T (2008) Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem Biophys Res Commun 365(2):279–284. doi:10.1016/j.bbrc.2007.10.182
Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, Varner JA (2011) Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res 71(22):6965–6975. doi:10.1158/0008-5472.CAN-11-0588
Fischer C, Mazzone M, Jonckx B, Carmeliet P (2008) FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8(12):942–956. doi:nrc2524[pii]10.1038/nrc2524
Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K, Schafer R, Stanley ER, Hofbauer R (2002) Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res 62(18):5317–5324
Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP (2005) Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 65(8):3437–3446. doi:10.1158/0008-5472.CAN-04-4262
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257. doi:10.1038/35025220
Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7(4):452–464. doi:10.1215/S1152851705000232
Gerhardt H, Betsholtz C (2003) Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314(1):15–23. doi:10.1007/s00441-003-0745-x
Gerhardt H, Semb H (2008) Pericytes: gatekeepers in tumour cell metastasis? J Mol Med (Berl) 86(2):135–144. doi:10.1007/s00109-007-0258-2
Kalluri R (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3(6):422–433. doi:10.1038/nrc1094
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401. doi:10.1038/nrc1877
O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J (1997) Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88(2):277–285
Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E (1999) Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest 103(2):159–165. doi:10.1172/JCI5028
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D (2003) Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 111(9):1287–1295. doi:10.1172/JCI17929
Bono AV, Pannellini T, Liberatore M, Montironi R, Cunico SC, Cheng L, Sasso F, Musiani P, Iezzi M (2010) Sorafenib's inhibition of prostate cancer growth in transgenic adenocarcinoma mouse prostate mice and its differential effects on endothelial and pericyte growth during tumor angiogenesis. Anal Quant Cytol Histol 32(3):136–145
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62. doi:10.1126/science.1104819
Jain RK, Booth MF (2003) What brings pericytes to tumor vessels? J Clin Invest 112(8):1134–1136. doi:10.1172/JCI20087
Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C (2002) Analysis of mural cell recruitment to tumor vessels. Circulation 105(1):112–117
Allt G, Lawrenson JG (2001) Pericytes: cell biology and pathology. Cells Tissues Organs 169(1):1–11
Berthod F, Symes J, Tremblay N, Medin JA, Auger FA (2012) Spontaneous fibroblast-derived pericyte recruitment in a human tissue-engineered angiogenesis model in vitro. J Cell Physiol 227(5):2130–2137. doi:10.1002/jcp.22943
Aghi M, Chiocca EA (2005) Contribution of bone marrow-derived cells to blood vessels in ischemic tissues and tumors. Mol Ther 12(6):994–1005. doi:10.1016/j.ymthe.2005.07.693
Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85(3):221–228
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275(5302):964–967
Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J (2009) Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther 17(1):183–190. doi:10.1038/mt.2008.229
Katyshev V, Dore-Duffy P (2012) Pericyte coculture models to study astrocyte, pericyte, and endothelial cell interactions. Methods Mol Biol 814:467–481. doi:10.1007/978-1-61779-452-0_31
Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA (2004) Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res 64(23):8492–8495. doi:10.1158/0008-5472.CAN-04-1708
Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K (2000) Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408(6808):92–96. doi:10.1038/35040568
Lamagna C, Bergers G (2006) The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol 80(4):677–681. doi:10.1189/jlb.0506309
Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G (2005) PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 7(9):870–879. doi:10.1038/ncb1288
Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P (2004) Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104(7):2084–2086. doi:10.1182/blood-2004-01-03362004-01-0336
Killingsworth MC, Wu X (2011) Vascular pericyte density and angiogenesis associated with adenocarcinoma of the prostate. Pathobiology 78(1):24–34. doi:10.1159/000322739
Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB (2001) NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 222(2):218–227. doi:10.1002/dvdy.1200
Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126(14):3047–3055
Ostman A, Heldin CH (2007) PDGF receptors as targets in tumor treatment. Adv Cancer Res 97:247–274. doi:10.1016/S0065-230X(06)97011-0
Tallquist M, Kazlauskas A (2004) PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15(4):205–213. doi:10.1016/j.cytogfr.2004.03.003S1359610104000139
Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15(1):21–34. doi:10.1016/j.ccr.2008.12.004
Lindahl P, Johansson BR, Leveen P, Betsholtz C (1997) Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277(5323):242–245
Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes HP, Shani M, Fassler R, Betsholtz C (2002) Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J 21(16):4307–4316
Soriano P (1994) Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8(16):1888–1896
McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, Bucana C, Ellis LM (2007) Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest 117(8):2114–2122. doi:10.1172/JCI31334
Iivanainen E, Nelimarkka L, Elenius V, Heikkinen SM, Junttila TT, Sihombing L, Sundvall M, Maatta JA, Laine VJ, Yla-Herttuala S, Higashiyama S, Alitalo K, Elenius K (2003) Angiopoietin-regulated recruitment of vascular smooth muscle cells by endothelial-derived heparin binding EGF-like growth factor. FASEB J 17(12):1609–1621. doi:10.1096/fj.02-0939com
Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD (1996) Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87(7):1171–1180. doi:S0092-8674(00)81813-9
Jones N, Iljin K, Dumont DJ, Alitalo K (2001) Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol 2(4):257–267. doi:10.1038/3506700535067005
Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8(16):1897–1909
Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC (2006) Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood 108(4):1260–1266. doi:10.1182/blood-2005-09-012807
Feng Y, vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, Wagner P, Lin J, Deutsch U, Hammes HP (2007) Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost 97(1):99–108. doi:10.1160/TH06-05-0277
Ma J, Wang Q, Fei T, Han JD, Chen YG (2007) MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood 109(3):987–994. doi:10.1182/blood-2006-07-036400
Stratmann A, Risau W, Plate KH (1998) Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol 153(5):1459–1466. doi:10.1016/S0002-9440(10)65733-1
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277(5322):55–60
Huang Y, Song N, Ding Y, Yuan S, Li X, Cai H, Shi H, Luo Y (2009) Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res 69(19):7529–7537. doi:10.1158/0008-5472.CAN-08-4382
Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P (2002) Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 21(7):1743–1753. doi:10.1093/emboj/21.7.1743
Lebrin F, Deckers M, Bertolino P, Ten Dijke P (2005) TGF-beta receptor function in the endothelium. Cardiovasc Res 65(3):599–608. doi:10.1016/j.cardiores.2004.10.036
Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP (1999) Defective angiogenesis in mice lacking endoglin. Science 284(5419):1534–1537
Oshima M, Oshima H, Taketo MM (1996) TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev Biol 179(1):297–302. doi:10.1006/dbio.1996.0259S0012-1606(96)90259-6
Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX (1999) Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126(8):1571–1580
Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest 106(8):951–961. doi:10.1172/JCI10905
Van Brocklyn JR, Graler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S (2000) Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood 95(8):2624–2629
McGuire PG, Rangasamy S, Maestas J, Das A (2011) Pericyte-derived sphingosine 1-phosphate induces the expression of adhesion proteins and modulates the retinal endothelial cell barrier. Arterioscler Thromb Vasc Biol 31(12):e107–e115. doi:10.1161/ATVBAHA.111.235408
Baluk P, Hashizume H, McDonald DM (2005) Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev 15(1):102–111. doi:10.1016/j.gde.2004.12.005
Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM (2002) Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol 160(3):985–1000. doi:10.1016/S0002-9440(10)64920-6
Gee MS, Procopio WN, Makonnen S, Feldman MD, Yeilding NM, Lee WM (2003) Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am J Pathol 162(1):183–193. doi:10.1016/S0002-9440(10)63809-6
Shaheen RM, Tseng WW, Davis DW, Liu W, Reinmuth N, Vellagas R, Wieczorek AA, Ogura Y, McConkey DJ, Drazan KE, Bucana CD, McMahon G, Ellis LM (2001) Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res 61(4):1464–1468
Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM (2006) Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 116(10):2610–2621. doi:10.1172/JCI24612
Lu C, Shahzad MM, Moreno-Smith M, Lin YG, Jennings NB, Allen JK, Landen CN, Mangala LS, Armaiz-Pena GN, Schmandt R, Nick AM, Stone RL, Jaffe RB, Coleman RL, Sood AK (2010) Targeting pericytes with a PDGF-B aptamer in human ovarian carcinoma models. Cancer Biol Ther 9(3):176–182. doi:10.4161/cbt.9.3.10635
Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM (2007) Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res 67(15):7358–7367. doi:10.1158/0008-5472.CAN-07-0293
Acknowledgements
This work is supported in part by the General Programs of the National Natural Science Foundation of China (No. 81071742, No. 81171998 and No. 81171999) and the Doctoral Fund of the New Teacher Program of Ministry of Education of China (No. 20110002120039).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yanping Ding and Nan Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ding, Y., Song, N. & Luo, Y. Role of Bone Marrow-Derived Cells in Angiogenesis: Focus on Macrophages and Pericytes. Cancer Microenvironment 5, 225–236 (2012). https://doi.org/10.1007/s12307-012-0106-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-012-0106-y