Abstract
Background
The cuff tendon that is most prone to full-thickness rotator cuff tears is the supraspinatus (SSP). Arthroscopic SSP repair ensures good to satisfactory mid- to long-term clinical outcomes. However, the intense postoperative pain reduces rehabilitation compliance and is cause of patient dissatisfaction. Many natural compounds act by inhibiting inflammatory pathways in a similar way to anti-inflammatory drugs
Materials and methods
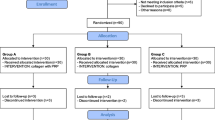
This was a prospective randomized trial designed to assess the analgesic effect of a dietary supplement (DS) containing Boswellia serrata and Curcuma longa in a population of subjects with full-thickness SSP tendon tear treated by arthroscopy. Three weeks before surgery, patients were randomized to receive Tendisulfur® (group T) or a placebo (group P) for 2 months. The primary outcome measure was subjective VAS pain. Secondary outcomes measures were Constant–Murley score simple shoulder test, and patient global assessment (PGA) scores. Patients were assessed immediately at baseline and subsequently at 1, 2, 4, 6, 8, 12, and 24 weeks.
Results
Stratification of pain scores and subscores demonstrated significantly lower overall pain scores in group T versus group P at 1 week (p = 0.0477), and lower but not significantly different scores on week 2 (p = 0.0988); at subsequent time points, differences were not significant (p > 0.05). PGA scores were good in all subjects.
Conclusions
In conclusion, this study provides objective data on the effect of a DS containing natural substances, added to standard analgesics, on postoperative RC pain. DS alleviated short and partially mid-term pain, while long-term pain was unchanged. This limitation can probably be addressed by a dosage increase over the first 4 weeks and by extending treatment by 1 or 2 months.




Similar content being viewed by others
References
Constant CR, Murley AH (1987) A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 214:160–164
Yamaguchi K, Ditsios K, Middleton WD et al (2006) The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am 88:1699–1704. doi:10.2106/JBJS.E.00835
Keener JD, Steger-May K, Stobbs G, Yamaguchi K (2010) Asymptomatic rotator cuff tears: patient demographics and baseline shoulder function. J Shoulder Elbow Surg 19:1191–1198. doi:10.1016/j.jse.2010.07.017
Hashimoto T, Nobuhara K, Hamada T (2003) Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res 415:111–120. doi:10.1097/01.blo.0000092974.12414.22
Lake SP, Miller KS, Elliott DM, Soslowsky LJ (2010) Tensile properties and fiber alignment of human supraspinatus tendon in the transverse direction demonstrate inhomogeneity, nonlinearity, and regional isotropy. J Biomech 43:727–732. doi:10.1016/j.jbiomech.2009.10.017
Seitz AL, McClure PW, Finucane S et al (2011) Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon) 26:1–12. doi:10.1016/j.clinbiomech.2010.08.001
Harvie P, Ostlere SJ, Teh J et al (2004) Genetic influences in the aetiology of tears of the rotator cuff. Sibling risk of a full-thickness tear. J Bone Joint Surg Br 86:696–700
Yamaguchi K, Tetro AM, Blam O et al. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg 10:199–203. doi: 10.1067/mse.2001.113086
Millett PJ, Warth RJ (2014) Posterosuperior rotator cuff tears: classification, pattern recognition, and treatment. J Am Acad Orthop Surg 22:521–534. doi:10.5435/JAAOS-22-08-521
MacKechnie MAK, Chahal J, Wasserstein D et al (2014) Repair of full-thickness rotator cuff tears in patients aged younger than 55 years. Arthroscopy 30:1366–1371. doi:10.1016/j.arthro.2014.05.011
Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, Diercks RL (2014) Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg 23:1073–1080. doi:10.1016/j.jse.2014.01.001
Millett PJ, Warth RJ, Dornan GJ et al (2014) Clinical and structural outcomes after arthroscopic single-row versus double-row rotator cuff repair: a systematic review and meta-analysis of level I randomized clinical trials. J Shoulder Elbow Surg 23:586–597. doi:10.1016/j.jse.2013.10.006
Kim C-W, Kim J-H, Kim D-G (2014) The factors affecting pain pattern after arthroscopic rotator cuff repair. Clin Orthop Surg 6:392–400. doi:10.4055/cios.2014.6.4.392
Talalay P (2001) The importance of using scientific principles in the development of medicinal agents from plants. Acad Med 76:238–247
Rehman Q, Sack KE (1999) When to try COX-2-specific inhibitors. Safer than standard NSAIDs in some situations. Postgrad Med 106:95–97, 101–102, 105–106
Hostanska K, Daum G, Saller R (2002) Cytostatic and apoptosis-inducing activity of boswellic acids toward malignant cell lines in vitro. Anticancer Res 22:2853–62
Fitzgerald GA (2004) Coxibs and cardiovascular disease. N Engl J Med 351:1709–1711. doi:10.1056/NEJMp048288
Harris WS, Von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 39:212–220. doi:10.1016/j.ypmed.2004.02.030
Mix KS, Mengshol JA, Benbow U et al (2001) A synthetic triterpenoid selectively inhibits the induction of matrix metalloproteinases 1 and 13 by inflammatory cytokines. Arthritis Rheum 44:1096–1104. doi:10.1002/1529-0131(200105)44:5<1096:AID-ANR190>3.0.CO;2-6
Almekinders LC (1999) Anti-inflammatory treatment of muscular injuries in sport. An update of recent studies. Sports Med (Auckland, NZ) 28:383–388
Miller MJS, Ahmed S, Bobrowski P, Haqqi TM (2006) The chrondoprotective actions of a natural product are associated with the activation of IGF-1 production by human chondrocytes despite the presence of IL-1beta. BMC Complement Altern Med 6:13. doi:10.1186/1472-6882-6-13
Maroon JC, Bost JW, Maroon A (2010) Natural anti-inflammatory agents for pain relief. Surg Neurol Int 1:80. doi:10.4103/2152-7806.73804
Maroon JC, Bost JW, Borden MK et al (2006) Natural antiinflammatory agents for pain relief in athletes. Neurosurg Focus 21:E11
Malavolta EA, Assunção JH, de Araujo AO et al (2014) Full-thickness supraspinatus tendon tears: correlation of findings by arthroscopy and magnetic resonance imaging. Int Orthop. doi:10.1007/s00264-014-2490-z
Bryant L, Shnier R, Bryant C, Murrell GAC (2002) A comparison of clinical estimation, ultrasonography, magnetic resonance imaging, and arthroscopy in determining the size of rotator cuff tears. J Shoulder Elbow Surg 11:219–24. doi: 10.1067/mse.2002.121923
Snyder SJ (2003) Arthroscopic classification of rotator cuff lesions and surgical decision making. Shoulder arthroscopy, 2nd edn. Lippincott Williams & Wilkins, Philadelphia, pp 201–207
Jobe FW, Moynes DR (1982) Delineation of diagnostic criteria and a rehabilitation program for rotator cuff injuries. Am J Sports Med 10:336–339
Fuchs B, Weishaupt D, Zanetti M et al (1999) Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 8:599–605
Godfrey J, Hamman R, Lowenstein S et al. Reliability, validity, and responsiveness of the simple shoulder test: psychometric properties by age and injury type. J Shoulder Elbow Surg 16:260–267. doi: 10.1016/j.jse.2006.07.003
Boss AP, Maurer T, Seiler S et al. Continuous subacromial bupivacaine infusion for postoperative analgesia after open acromioplasty and rotator cuff repair: preliminary results. J Shoulder Elbow Surg 13:630–634. doi: 10.1016/S1058274604001363
Cho C-H, Song K-S, Min B-W et al (2011) Multimodal approach to postoperative pain control in patients undergoing rotator cuff repair. Knee Surg Sports Traumatol Arthrosc 19:1744–1748. doi:10.1007/s00167-010-1294-y
Cho NS, Ha JH, Rhee YG (2007) Patient-controlled analgesia after arthroscopic rotator cuff repair: subacromial catheter versus intravenous injection. Am J Sports Med 35:75–79. doi:10.1177/0363546506291632
Oh JH, Kim WS, Kim JY et al (2007) Continuous intralesional infusion combined with interscalene block was effective for postoperative analgesia after arthroscopic shoulder surgery. J Shoulder Elbow Surg 16:295–299. doi: 10.1016/j.jse.2006.04.015
Arndt J, Clavert P, Mielcarek P et al (2012) Immediate passive motion versus immobilization after endoscopic supraspinatus tendon repair: a prospective randomized study. Orthop Traumatol Surg Res 98:S131–S138. doi:10.1016/j.otsr.2012.05.003
Chang K-V, Hung C-Y, Han D-S et al (2014) Early versus delayed passive range of motion exercise for arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med. doi:10.1177/0363546514544698
Conti M, Garofalo R, Delle Rose G et al (2009) Post-operative rehabilitation after surgical repair of the rotator cuff. La Chirurgia degli organi di movimento 93(Suppl 1):S55–S63. doi:10.1007/s12306-009-0003-9
Denard PJ, Lädermann A, Burkhart SS (2011) Prevention and management of stiffness after arthroscopic rotator cuff repair: systematic review and implications for rotator cuff healing. Arthroscopy 27:842–848. doi:10.1016/j.arthro.2011.01.013
Garofalo R, Conti M, Notarnicola A et al (2010) Effects of one-month continuous passive motion after arthroscopic rotator cuff repair: results at 1-year follow-up of a prospective randomized study. Musculoskelet Surg 94(Suppl 1):S79–S83. doi:10.1007/s12306-010-0058-7
Notarnicola A, Pesce V, Vicenti G et al (2012) SWAAT study: extracorporeal shock wave therapy and arginine supplementation and other nutraceuticals for insertional Achilles tendinopathy. Adv Ther 29:799–814. doi:10.1007/s12325-012-0046-4
Gumina S, Passaretti D, Gurzì MD, Candela V (2012) Arginine l-alpha-ketoglutarate, methylsulfonylmethane, hydrolyzed type I collagen and bromelain in rotator cuff tear repair: a prospective randomized study. Curr Med Res Opin 28:1767–1774. doi:10.1185/03007995.2012.737772
Arafa NM, Hamuda HM, Melek ST, Darwish SK (2013) The effectiveness of Echinacea extract or composite glucosamine, chondroitin and methyl sulfonyl methane supplements on acute and chronic rheumatoid arthritis rat model. Toxicol Ind Health 29:187–201. doi:10.1177/0748233711428643
Joksimovic N, Spasovski G, Joksimovic V et al (2012) Efficacy and tolerability of hyaluronic acid, tea tree oil and methyl-sulfonyl-methane in a new gel medical device for treatment of haemorrhoids in a double-blind, placebo-controlled clinical trial. Updates Surg 64:195–201. doi:10.1007/s13304-012-0153-4
Kim LS, Axelrod LJ, Howard P et al (2006) Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial. Osteoarthritis Cartilage 14:286–294. doi:10.1016/j.joca.2005.10.003
Ebisuzaki K (2003) Aspirin and methylsulfonylmethane (MSM): a search for common mechanisms, with implications for cancer prevention. Anticancer Res 23:453–458
Alam SS, Layman DL (1983) Dimethyl sulfoxide inhibition of prostacyclin production in cultured aortic endothelial cells. Ann NY Acad Sci 411:318–320
Beilke MA, Collins-Lech C, Sohnle PG (1987) Effects of dimethyl sulfoxide on the oxidative function of human neutrophils. J Lab Clin Med 110:91–96
Morton JI, Siegel B V (1986) Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY) 183:227–230
Pavelká K, Gatterová J, Olejarová M et al (2002) Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 162:2113–2123
Usha PR, Naidu MUR (2004) Randomised, double-blind, parallel, placebo-controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clin Drug Investig 24:353–363
Klein MB, Yalamanchi N, Pham H et al (2002) Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg 27:615–620
Provenzano PP, Vanderby R (2006) Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol 25:71–84. doi:10.1016/j.matbio.2005.09.005
Pauly S, Klatte F, Strobel C et al (2010) Characterization of tendon cell cultures of the human rotator cuff. Eur Cells Mater 20:84–97
Shakibaei M, Buhrmann C, Mobasheri A (2011) Anti-inflammatory and anti-catabolic effects of TENDOACTIVE® on human tenocytes in vitro. Histol Histopathol 26:1173–1185
Ahmadzadeh H, Connizzo BK, Freedman BR et al (2013) Determining the contribution of glycosaminoglycans to tendon mechanical properties with a modified shear-lag model. J Biomech 46:2497–2503. doi:10.1016/j.jbiomech.2013.07.008
Scott JE, Hughes EW (1986) Proteoglycan-collagen relationships in developing chick and bovine tendons. Influence of the physiological environment. Connect Tissue Res 14:267–278
Attia M, Scott A, Duchesnay A et al (2012) Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res 30:61–71. doi:10.1002/jor.21479
Legerlotz K, Riley GP, Screen HRC (2013) GAG depletion increases the stress-relaxation response of tendon fascicles, but does not influence recovery. Acta Biomater 9:6860–6866. doi:10.1016/j.actbio.2013.02.028
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012. doi:10.1056/NEJM199312303292706
Förstermann U, Closs EI, Pollock JS et al (1994) Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23:1121–1131
Bode-Böger SM, Böger RH, Galland A et al (1998) l-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol 46:489–497
Murrell GA, Szabo C, Hannafin JA et al (1997) Modulation of tendon healing by nitric oxide. Inflamm Res 46:19–27
Ammon HPT (2006) Boswellic acids in chronic inflammatory diseases. Planta Med 72:1100–1116. doi:10.1055/s-2006-947227
Syrovets T, Büchele B, Krauss C et al (2005) Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J Immunol (Baltimore, Md: 1950) 174:498–506
Syrovets T, Gschwend JE, Büchele B et al (2005) Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J Biol Chem 280:6170–6180. doi:10.1074/jbc.M409477200
Bharti AC, Donato N, Singh S, Aggarwal BB (2003) Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaB alpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 101:1053–1062. doi:10.1182/blood-2002-05-1320
Bharti AC, Aggarwal BB (2002) Chemopreventive agents induce suppression of nuclear factor-kappaB leading to chemosensitization. Ann NY Acad Sci 973:392–395
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23:363–398
Buhrmann C, Mobasheri A, Matis U, Shakibaei M (2010) Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther 12:R127. doi:10.1186/ar3065
Shakibaei M, John T, Schulze-Tanzil G et al (2007) Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol 73:1434–1445. doi:10.1016/j.bcp.2007.01.005
Chrubasik S, Eisenberg E, Balan E et al (2000) Treatment of low back pain exacerbations with willow bark extract: a randomized double-blind study. Am J Med 109:9–14
Freedman JE, Parker C, Li L et al (2001) Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 103:2792–2798
Acknowledgments
The authors are grateful to Word Designs (www.silviamodena.com) for the language revision.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merolla, G., Dellabiancia, F., Ingardia, A. et al. Co-analgesic therapy for arthroscopic supraspinatus tendon repair pain using a dietary supplement containing Boswellia serrata and Curcuma longa: a prospective randomized placebo-controlled study. Musculoskelet Surg 99 (Suppl 1), 43–52 (2015). https://doi.org/10.1007/s12306-015-0364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-015-0364-1