Abstract
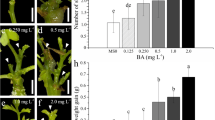
Broccoli (Brassica oleracea L. var. italica) is an important, nutritionally rich vegetable crop, but severely affected by environmental stresses, pests and diseases which cause massive yield and quality losses. Genetic manipulation is becoming an important method for broccoli improvement. In the present study, a reproducible and highly efficient protocol for obtaining organogenesis from hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica cv. Solan green head) has been developed. Hypocotyl and cotyledon explants were used from 10 to 12 days old aseptically grown seedlings whereas leaf and petiole explants were excised from 18 to 20 days old green house grown seedlings and surface sterilized. These explants were cultured on shoot induction medium containing different concentration and combination of BAP and NAA. High efficiency shoot regeneration has been achieved in hypocotyl (83.33 %), cotyledon (90.11 %), leaf (62.96 %) and petiole (91.10 %) explants on MS medium supplemented with 3.5 mg/l BAP + 0.019 mg/l NAA 2.5 mg/l BAP + 0.5 mg/l NAA, 4.0 mg/l BAP + 0.5 mg/l NAA and 4.5 mg/l BAP + 0.019 mg/l NAA respectively. Petiole explants showed maximum shoot regeneration response as compared to other explants. MS medium supplemented with 0.10 mg/l NAA was found best for root regeneration (100 %) from in vitro developed shoots. The regenerated complete plantlets were transferred to the pots containing cocopeat and successfully acclimatized. This optimized regeneration protocol can be efficiently used for genetic transformation in broccoli. This is the first comparative report on multiple shoot induction using four different types of explants viz. hypocotyl, cotyledon, leaf and petiole.


Similar content being viewed by others
Abbreviations
- MS :
-
Murashige & Skoog
- NAA :
-
Naphthalene acetic acid
- BAP- 6 :
-
Benzyl aminopurine
- IAA :
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
References
Amita G, Aggarwal G, Srivastava DK (2012) Plant regeneration, genetic transformation and expression of foreign gene cotyledon and petiole tissues of cucumber (Cucumis sativus L. cv. K-75). Adv Appl Res 4(1):46–52
Arnison PG, Donaldson P, Jackson A, Semple C, Keller W (1990) Genotype-specific response of cultured broccoli (Brassica oleracea var. italica) anthers to cytokinins. Plant Cell Tis Org Cul 20:217–228
Bano R, Khan MH, Khan RS, Rashid H, Swat ZA (2010) Development of an efficient regeneration protocol for three genotypes of Brassica juncea. Pak J Bot 42(2):963–969
Cao J, Earle ED (2003) Transgene expression in broccoli (Brassica oleracea var. italica) clones propagated in vitro via leaf explants. Plant Cell Rep 21:789–796
Chakrabarty R, Viswakarma N, Bhat SR, Kirti PB, Singh BD, Chopra VL (2002) Agrobacterium-mediated transformation of cauliflower: optimization of protocol and development of Bt-transgenic cauliflower. J Biosci 27:495–502
Christey MC, Earle ED (1991) Regeneration of Brassica oleracea from peduncle explants. Hortic Sci 26:1069–1072
Compton ME, Gray DJ (1993) Shoot organogenesis and plant regeneration from cotyledons of diploid, triploid and tetraploid watermelon. J Am Soc Hortic Sci 118:151–157
Dhir R, Shekhawat GS (2014) In vitro propagation using transverse thin cell layer culture and homogeneity assessment in Ceropegia bulbosa Roxb. J Plant Growth Regul 33(4):820–830
Dong JZ, Jia SR (1991) High efficiency plant regeneration from cotyledons of watermelon (Citrullus vulgaris Schard). Plant Cell Rep 9:858–863
George EF, Hall MA, Klerk GJD (2008) Plant propagation by tissue culture. The background, 3rd edn. Springer Publisher Dordrecht, London
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley and Sons, New York
Huang K, Qiuyun W, Juncleng L, Zheng J (2011) Optimization of plant regeneration from broccoli. Afr J Biotechnol 10(20):4081–4085
Jana S, Shekhawat GS (2010) Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens. Acta Physiol Plant 33:305–311
Kim JH, Botella JR (2002) Callus induction and plant regeneration from broccoli (Brassica oleracea var. italica) for transformation. J Plant Biochem 45(3):177–181
Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB (2007) Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst 99(15):1200–1209
Lazzeri PA, Dunwell JM (1986) In vitro regeneration from seedling organs of Brassica oleracea var. italica Plenck cv. Green comet. I. Effect of plant growth regulators. Ann Bot 58(5):689–697
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Puddephat IJ, Robinson HT, Fenning TM, Barbara DJ, Morton A, Pink DAC (2001) Recovery of phenotypically normal transgenic plants of Brassica oleracea L. var. italica upon Agrobacterium rhizogenes-mediated co-transformation and selection of transformed hairy roots by GUS assay. Mol Breed 7(3):229–242
Qin Y, Li HL, Guo YD (2006) High frequency embryogenesis, regeneration of broccoli (Brassica oleracea var. italica) and analysis of genetic stability by RAPD. Sci Hortic 111:203–208
Rajicic TS, D Stevanovic, R Djordjevic, M Veelickovic, Z Susic (2002) Maintenance of Prospective Cabbage Brassica oleracea var. capitata) Lines by Micropropagation. Acta Hortic. 579: II Balkan Symposium onVegetables and Potatoes.
Rani T, Yadav RC, Yadav NR, Asha R, Singh D (2013) Genetic transformation in oilseed brassicas: a review. Indian J Agric Sci 83(4):367–373
Ravanfar SA, Aziz MA, Kadir MA, Rashid AA, Haddadi (2011) In vitro shoot regeneration and acclimatization of Brassica oleracea var. italica cv. Green marvel. Afr J Biotechnol 10(29):5614–5619
Ravanfar SA, Aziz MA, Kadir MA, Rashid AA, Sirchi MHT (2009) Plant regeneration of Brassica oleracea var. italica (broccoli) cv. Green marvel was affected by plant growth regulators. Afr J Biotechnol 8(11):2523–2528
Robertson D, Earle ED (1986) Plant regeneration from leaf protoplasts of Brassica oleracea L. var. italica. Plant Cell Rep 5(1):61–64
Sharma S, Gambhir G, Srivastava DK (2014) High frequency organogenesis in cotyledon and hypocotyls explants of cabbage (Brassica oleracea L. var. capitata). Nat Acad Sci Lett 37:5–12
Sharma S, Sharma C, Srivastava DK (2012) Plant regeneration genetic transformation and gene expression in in vitro tissues of cauliflower (Brassica oleracea L. var botrytis). Bioinfolet 9(4B):760–764
Srivastava DK, Andrianov VM, Piruzian ES (1989) Tissue culture and plant regeneration of watermelon (Citrullus vulgaris Schard cv. Melitopulski). Plant Cell Rep 8:300–302
Srivastava DK, Andrianov VM, Piruzian ES (1991a) Regeneration and genetic transformation studies in watermelon (Citrullus vulgaris L. cv. Melitopolski). In: Horticulture- New Technologies and Applications, pp. 127–130 (Parkash J and Pierika RL M, eds.)
Srivastava DK, Kolgonova TV, Mett VL, Piruzian ES (1991b) Genetic transformation of cotton (Gossypium hirsutum L. cv. 108-F). Acta Hortic 289:263–264
Srivastava V, Reddy AS, Guha MS (1988) Transformation and regeneration of Brassica oleracea mediated by an oncogenic Agrobacterium tumefaciens. Plant Cell Rep 7:504–507
Vandemoortele JL, Billiard JP, Boucaud J, Gaspar T (1999) Evidence for an interaction between basal medium and plant growth regulators during adventitious or axillary shoot formation of cauliflower. In Vitro Cell Dev Biol Plant 35:13–17
Yang JL, Seong ES, Kim MJ, Ghimire BK, Kang WH, Chang YY, Cheng HL (2010) Direct somatic embryogenesis from pericycle cell of broccoli (Brassica oleracea L. var. italica) root explants. Plant Cell Tissue Organ Cult 100:49–58
Zhang FL, Takahata Y, Xu JB (1998) Medium and genotype factors influencing the regeneration from cotyledonary explants of Chinese cabbage (Brassica compestris L. ssp. Pekinensis). Plant Cell Rep 17:780–786
Acknowledgments
We are grateful to the Professor and Head, Department of Vegetable Science, Dr. Y. S. Parmar University of Horticulture and Forestry- Nauni, Solan for providing us the germplasm of broccoli cv. Solan green head. The senior author (P.K.) thankfully acknowledge the award of DST INSPIRE fellowship New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P., Srivastava, D.K. High frequency organogenesis in hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Physiol Mol Biol Plants 21, 279–285 (2015). https://doi.org/10.1007/s12298-015-0282-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-015-0282-6