Abstract
This study characterized Pokkali-derived quantitative trait loci (QTLs) for seedling stage salinity tolerance in preparation for use in marker-assisted breeding. An analysis of 100 SSR markers on 140 IR29/Pokkali recombinant inbred lines (RILs) confirmed the location of the Saltol QTL on chromosome 1 and identified additional QTLs associated with tolerance. Analysis of a series of backcross lines and near-isogenic lines (NILs) developed to better characterize the effect of the Saltol locus revealed that Saltol mainly acted to control shoot Na+/K+ homeostasis. Multiple QTLs were required to acquire a high level of tolerance. Unexpectedly, multiple Pokkali alleles at Saltol were detected within the RIL population and between backcross lines, and representative lines were compared with seven Pokkali accessions to better characterize this allelic variation. Thus, while the Saltol locus presents a complex scenario, it provides an opportunity for marker-assisted backcrossing to improve salt tolerance of popular varieties followed by targeting multiple loci through QTL pyramiding for areas with higher salt stress.
Similar content being viewed by others
Introduction
Salt stress is a major constraint across many rice production areas because of the high sensitivity of modern rice varieties. Salinity is particularly a major problem in coastal regions in the tropics where rice-based farming systems predominate. This is because of the intrusion of brackish water during the dry season and at the start of the wet season. Salt stress is also a worsening problem in inland areas because of the buildup of salinity as a consequence of excessive use of irrigation water with improper drainage coupled with the use of poor quality irrigation water (Ismail et al. 2010). Rice is considered sensitive to salinity, particularly during early vegetative and later at reproductive stages. Nonetheless, it is one of the few crops that can thrive on salt-affected soils because of its ability to grow well in standing water that can help leach salts from topsoil and is, therefore, recommended as an entry crop for desalinization of salt affected lands (Ismail et al. 2007; Singh et al. 2010).
Farmers have grown traditional rice landraces adapted to salt-affected areas for generations despite their numerous undesirable traits, including long duration, low yield, and poor grain quality. Some of these landraces possess remarkable tolerance to salt stress through a complex set of physiological mechanisms, including sodium exclusion, higher tissue tolerance by compartmenting salts into the apoplasts, effective sequestration of toxic salts into older tissues, stomatal responsiveness, and upregulating the antioxidant system during stress (Yeo and Flowers 1986; Ismail et al. 2007; Moradi and Ismail 2007). Moreover, tolerance during seedling stage seems to correlate poorly with tolerance during reproduction, suggesting different sets of traits are probably involved at each stage (Moradi et al. 2003). Despite this complexity, most salt-tolerant cultivars seem to posses only a few of these mechanisms, signifying the prospects for developing highly tolerant rice varieties through combining superior alleles of genes controlling these traits. Recent advances in molecular biology and genomics have led to a more detailed understanding of the genes and pathways involved in the salt stress response in rice, including those involved in ion transport and homeostasis, osmoregulation, and oxidative stress protection (Blumwald et al. 2000; Mäser et al. 2002; Garciadeblás et al. 2003; Chinnusamy et al. 2004; Horie and Schroeder 2004; Nakayama et al. 2005; Bohnert et al. 2006; Rodriguez-Navarro and Rubio 2006; Sahi et al. 2006; Martinoia et al. 2007; Munns and Tester 2008; Singh and Flowers 2010)
The vast genetic variability reported in rice in response to salinity makes it amenable to genetic manipulation to further enhance its tolerance (Akbar et al. 1972; Flowers and Yeo 1981). Breeders have long made use of the high level of salinity tolerance in landraces like Nona Bokra and Pokkali. However, negative characters in traditional varieties and the numerous and complex traits involved in salinity tolerance have presented challenges for conventional breeding to make significant progress and has led to increased interest in molecular breeding methods (Gregorio et al. 2002; Yamaguchi and Blumwald 2005; Ismail et al. 2007; Thomson et al. 2010). Mapping of quantitative trait loci (QTLs) can enable dissection of the genetic control of each tolerance mechanism, opening up the possibility of future efforts to develop varieties with improved salinity tolerance by precisely transferring QTLs into popular varieties and pyramiding multiple relevant QTLs for a particular stress-prone environment.
A number of mapping studies have identified QTLs associated with salinity tolerance in rice (Singh et al. 2007; Haq et al. 2010). For example, a study employing an F2:3 population between the tolerant indica landrace Nona Bokra with the susceptible japonica Koshihikari identified several QTLs controlling tolerance traits, including major QTLs for shoot K+ concentration on chromosome 1 (qSKC-1) and shoot Na+ concentration on chromosome 7 (qSNC-7; Lin et al. 2004). The SKC1 gene was subsequently cloned and found to encode a sodium transporter that helps control K+ homeostasis under salt stress (Ren et al. 2005). Likewise, to characterize the genetic components of salinity tolerance in the tolerant landrace Pokkali, a RIL population between the indica varieties IR29 and Pokkali was developed at IRRI and used in a QTL study using AFLP genotyping on a set of 38 tolerant and 42 sensitive RILs (Gregorio 1997). A major QTL associated with the Na–K ratio and seedling-stage salinity tolerance, named Saltol, was identified on chromosome 1, along with a number of minor QTLs on other chromosomes. Subsequently, RFLP and SSR markers were added to the Saltol region, and a test of 54 RILs in a hydroponic screen at the seedling stage revealed that this QTL explained 43% of the variation for seedling shoot Na–K ratio in this population (Bonilla et al. 2002).
One highly salt tolerant RIL from this population, FL478 (IR 66946-3R-178-1-1), has been promoted as an improved donor for breeding programs, as it has a high level of seedling stage salinity tolerance and is photoperiod insensitive, shorter and flowers earlier than the original Pokkali landrace. Several global gene expression profiling studies have investigated transcriptional differences between the susceptible IR29 compared with FL478, revealing the up-regulation of genes in FL478 under salt stress for ion transport and cell wall-related genes (Walia et al. 2005, 2009), while differential expression was observed in roots for cation transport proteins (Senadheera et al. 2009) and kinases and phosphatases (Senadheera and Maathuis 2009). Furthermore, an analysis of single feature polymorphisms in the Saltol region suggested that FL478 contained a <1 Mb DNA fragment from Pokkali at 10.6–11.5 Mb on chromosome 1, flanked by IR29 alleles (Kim et al. 2009).
While genetic and physiological studies have often employed the traditional variety Pokkali, in reality, there are many different accessions of pokkali-type rice varieties, as the name pokkali refers to a system of rice cultivation under saline conditions characterized by a high level of partial flooding and rotated with shrimp farming along the coast of Kerala, India (Shylaraj and Sasidharan 2005). Thus, there are many Pokkali type cultivars from different sources, including over a dozen accessions named “Pokkali” or “Pokkalian” held in the T.T. Chang Genetic Resources Center at IRRI (http://www.iris.irri.org/). Although the pokkali-type rice varieties potentially have useful genetic variation for salt tolerance, little work has been done to investigate the genetic diversity between different Pokkali accessions to identify the best donors for breeding. Furthermore, the existence of these multiple Pokkali accessions complicated their use in breeding as well as in QTL mapping without proper characterization.
The objectives of the current study were to (1) update the IR29/Pokkali QTL study with SSR markers to better define the locations of Pokkali-derived QTLs for seedling stage salt tolerance, (2) confirm the effect and location of Saltol using NILs, (3) compare the Pokkali alleles in the mapping populations with those of diverse Pokkali accessions, and (4) develop a marker-assisted backcrossing package for Saltol to rapidly develop varieties with improved tolerance incorporating this locus.
Results
QTLs identified using the IR29/Pokkali RIL population
A previous QTL study used AFLP markers on 80 IR29/Pokkali RILs selected to represent phenotypic extremes (Gregorio 1997), while a follow-up study focused on chromosome 1 to further define the position of the Saltol QTL (Bonilla et al. 2002). To provide a more comprehensive view of Pokkali QTLs using SSR markers, we performed a new QTL study using 100 genome-wide SSRs on 140 RILs, including 64 of the extreme RILs plus 76 randomly selected lines from the original population of 276 RILs developed from the cross of Pokkali x IR29 (Gregorio 1997). During the SSR genotyping, a number of non-parental alleles were also observed: out of 140 RILs, 26 lines had greater than 10% non-parental alleles across the 100 SSRs. To test whether these potentially represented multiple Pokkali alleles, the QTL analysis was performed with the extra alleles converted to Pokkali alleles versus disregarding them as missing data. The QTL effects were consistently higher when the extra alleles were designated as Pokkali (data not shown), suggesting that the “non-parental” alleles may actually represent multiple alleles from heterozygous loci of the original Pokkali parent.
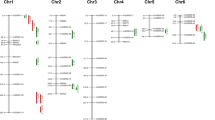
These 140 RILs were screened under controlled conditions in two experiments for a number of salt stress related traits (Supplementary Table 1). The RIL population was grown using a hydroponic system with a salt stress level of EC 12 dS m−1 using Yoshida nutrient solution (Yoshida et al. 1976) in Experiment 1, and up to an EC of 18 dS m−1 in Experiment 2. The population was evaluated for seedling stage salinity tolerance based on visual symptoms using IRRI’s standard evaluation system (SES) scores (IRRI 1996). In the first screening experiment, a significant QTL for shoot Na–K ratio was detected in the Saltol region on chromosome 1, with an LOD of 7.6 and R2 of 27%, along with a similar QTL for the root Na–K ratio (Table 1, Fig. 1). However, only a sub-threshold effect with a LOD of 2.3 was seen for the SES tolerance score at the Saltol locus (data not shown). A number of additional Pokkali-derived QTLs for tolerance traits were detected in other regions. Two QTLs for the SES tolerance score were detected in experiment 1: one on chromosome 4 along with seedling height and chlorophyll content and another on chromosome 9 along with shoot and root Na–K ratios (Table 1, Fig. 1). QTLs for root K+ concentration and root Na–K ratio were also detected on chromosome 6. In the second screening experiment under higher stress conditions, QTLs for SES tolerance score were detected on chromosome 3, along with chlorophyll content, and on chromosome 12 in the same region as QTLs for survival and chlorophyll content (Table 2, Fig. 1). A set of QTLs for survival and chlorophyll content were also detected on the bottom of chromosome 1, although the tolerance effect was derived from IR29 instead of Pokkali in this region. In addition, one region on chromosome 2 contained a cluster of Pokkali-derived QTLs from both experiments, including height, root K+ concentration, chlorophyll content, and survival (Tables 1 and 2, Fig. 1).
QTLs identified from a population of 140 IR29/Pokkali RILs using 100 SSR markers and 14 salinity tolerance traits measured at the seedling stage with a hydroponic system under controlled conditions. Significant QTLs (represented by black bars) were identified for seedling plant height (PH), shoot Na+ concentration (SNC), shoot K+ concentration (SKC), shoot Na–K ratio (SNK), root K+ concentration (RKC), root Na–K ratio (RNK), the visual tolerance score (SES), leaf chlorophyll content (CHL), and seedling survival (SUR).
Characterizing backcross lines and NILs to confirm the Saltol effect
A set of 39 BC3F5 IR29/Pokkali lines were previously developed at IRRI using Pokkali as the donor and IR29 as the recurrent parent. These backcross lines were analyzed with five SSRs between 11 and 15 Mb on chromosome 1 to assign categories based on having a homozygous Pokkali allele, heterozygous allele, or IR29 allele at the Saltol locus, along with 95 SSRs across the rest of the genome to detect background introgressions (Supplementary Table 2). Salinity tolerance traits were measured as with the RIL population. Overall the backcross lines were intermediate between the sensitive (IR29) and tolerant (FL478) checks, and differences were observed between the means of the Saltol versus non-Saltol backcross lines for SES, chlorophyll content, shoot Na–K ratio and root Na–K ratio (Fig. 2). The largest difference was observed for shoot Na–K ratio with an average of 1.2 (with Saltol) versus 1.7 (without Saltol), while the differences for the other traits were relatively small: an average SES score of 5.1 (with Saltol) versus 5.4 (without Saltol), a root Na–K ratio of 1.4 versus 1.8, and a chlorophyll content of 0.08 versus 0.06, respectively. However, the differences between Saltol lines and the sensitive parent IR29 were much larger for all traits except for chlorophyll content (Supplementary Table 2). Furthermore, the fact that even the non-Saltol lines had higher tolerance than the IR29 check indicated additional tolerance QTLs were still present in the Pokkali introgressions in the IR29 background.
a SES scores, b chlorophyll content, c shoot, and d root Na–K ratio of a set of 39 BC3F5 IR29/Pokkali lines analyzed with SSRs between 11 and 15 Mb on chromosome 1 (RM1287, RM10694, RM8094, RM10793, RM10864, and RM7075) to assign categories based on having a homozygous Pokkali allele (20 lines), heterozygous allele (four lines), or IR29 allele (15 lines) at the Saltol locus. Trait means with standard errors (vertical bars) are shown for each genotypic category along with the IR29 (sensitive) and FL478 (tolerant) checks across the four traits tested at the seedling stage using a hydroponic system at an EC of 12 dS m−1.
Since the IR29/Pokkali BC3F5 backcross lines did not show a large tolerance effect at Saltol, a new backcross population was developed using FL478 as the donor and IR29 as the recurrent parent. Seven FL478/IR29 BC3F4 backcross families along with additional BC3F5 IR29/Pokkali individuals were screened with markers to select for fixed lines representative of different Pokkali introgressions across the Saltol region. Selected individuals were selfed, and the progeny were genotyped with 38 SSR markers and screened for salinity tolerance (Supplementary Table 3). A comparison of the Pokkali introgressions across 16 selected backcross lines confirmed an intermediate tolerance effect from two different Pokkali alleles detected at the Saltol locus: the more common allele as seen in FL378 and FL540 and the distinct allele derived from FL478 (Fig. 3). Specifically, an IR29/Pokkali NIL with no detected background Pokkali introgressions (J19) had a SES score of 5.3 due to the Pokkali introgression at the Saltol locus, compared to the IR29 control which had an SES score of 7.7 in the same screen experiment. Likewise, FL478-derived backcross lines with a different Pokkali allele at the Saltol locus (J63, J64, J76, and J79) also showed increased tolerance, although these lines still had one or two background introgressions. The location of these Pokkali introgressions between 11 and 12.2 Mb supports the possibility that the Pokkali Saltol QTL may be controlled by the same gene as the SKC1 QTL located at 11.46 Mb, which was first characterized using the tolerant Nona Bokra allele (Ren et al. 2005). Several insertion–deletion markers near SKC1 were developed and used to track the specific SKC1 alleles in the different backcross lines. Furthermore, two backcross lines (J71 and J80) did not have a Pokkali allele at Saltol but showed high tolerance, presumably due to other Pokkali QTLs in the background (Fig. 3).
A set of 16 IR29/Pokkali and FL478/IR29 backcross lines and three RILs screened with 38 markers to define their Pokkali introgressions. The original QTL interval plots for the shoot Na–K ratio (solid line) and root Na–K ratio (dotted line) are shown on the left to indicate the location of the Saltol QTL peak. Additional markers were genotyped at the Saltol locus to more precisely define the introgression boundaries, as indicated by hash marks between RM1287 and RM7075, representing RM10694 (11 Mb), AP3206 (11.2 Mb), SKC1b (11.4 Mb), RM3412 (11.5 Mb), RM493 (12.2 Mb), RM10793 (12.5 Mb), RM10864, (14.2 Mb), and RM562 (14.6 Mb). The most common Pokkali introgressions are shown as lightly shaded boxes, the FL478 Saltol allele is shown as darkly shaded boxes, and two additional alleles derived from NIL-17 (J21) and NIL-30 (J38) are shown by striped boxes. The presence of background Pokkali introgressions is indicated by the chromosome numbers below each line (with several lines having numerous background introgressions, indicated as NUM), and the SES tolerance scores for each line are shown below each line for experiments under standard phytotron conditions (SES-1) and in growth chambers under high temperature conditions (SES-2), along with the Na–K ratio from the growth chamber experiment.
Subsequently, 140 FL478/IR29 BC3F4 lines were genotyped with 28 SSR markers, and 55 lines selected for target FL478 introgressions with a higher proportion of IR29 in the background were screened for tolerance in the phytotron. Five promising lines were backcrossed two more times to IR29, and 122 FL478/IR29 BC5F1 plants were genotyped with 63 SSR markers to more precisely define the Pokkali introgressions. Nine NILs with a single Pokkali introgression at the Saltol QTL on chromosome 1 and a clean IR29 background were identified and their progeny, along with the IR29 check and an FL478/IR29 BC5F2 family with multiple introgressions, were evaluated in a saline field in Iloilo, Philippines and under controlled condition in the field at IRRI. Although two of the Saltol NILs had higher yields than the IR29 check (line B6 with a yield of 1431 kg ha−1 and line D10 with a yield of 1258 kg ha−1, compared to the IR29 check at 1163 kg ha−1), the other lines did not outperform IR29, suggesting that both seedling and reproductive stage tolerance will need to be combined into the same lines to achieve higher yields under stress in saline fields (Supplementary Table 4) as has been suggested before (Moradi et al. 2003). However, one line with multiple Pokkali introgressions performed better in the field, with a yield of 1,964 kg ha−1, which indicates that other Pokkali-derived QTLs may help provide a higher level of tolerance for yield under saline field conditions. Comparing the yield under stress with that under non-stress irrigated conditions shows that under stress conditions line A18 yielded 35% of the control yield, while Saltol lines D10, D7 and B6 yielded 33%, 25%, and 24%, respectively, compared with the IR29 control that yielded 20% in stressed versus non-stressed fields (Supplementary Table 4).
Multiple Pokkali alleles detected across different RILs and NILs
During the initial SSR genotyping of the IR29/Pokkali QTL population, multiple Pokkali alleles were observed between RILs at certain loci. For example, at the marker RM8094 on chromosome 1, four of the RILs had a different Pokkali allele than the rest of the population: FL334, FL478, FL479, and FL481 (data not shown). Likewise, during the process of developing NILs for the Saltol locus, multiple Pokkali alleles were observed between different sets of backcross lines. This complicated the analysis of the Saltol locus and the subsequent development of markers for MABC; hence the following analysis was undertaken to resolve these problems. The majority of the IR29/Pokkali backcross lines and the RIL population shared a common Pokkali allele; however, the lines derived from FL478 had a distinct allele at Saltol. Furthermore, two slightly different alleles were also detected in the IR29/Pokkali backcross population: one represented by line J21 (derived from the NIL-17 family) and the other found in J38 (derived from the NIL-30 family) that did not seem to match the other IR29/Pokkali or FL478 backcross lines (Fig. 3). Although the marker haplotypes across the Saltol region for these two lines are different, line J21 shares the same allele at the gene-based marker SKC1b as the more common Pokkali allele, while the SKC1b allele at line J38 was the same as IR29 (Supplementary Table 3).
To further decipher the origin and characterize the relationships between these alleles and multiple Pokkali accessions in the germplasm collection, a comparison was performed with 17 markers across the Saltol region and 61 SSRs across the background on seven Pokkali genebank accessions (IRGC 8948, 15388, 15602, 15661, 26896, 28609, 108921) and IR29, FL478, NIL-17, and NIL-30 (Supplementary Table 5). The results showed that at the Saltol region, the FL478 allele was most similar to that of Pokkali accession IRGC 28609; however, the background markers did not show this relationship (Fig. 4). Moreover, it was confirmed that the Pokkali accessions being used for studies at UC Davis (Pokkali-UCD) and Dhaka University (Pokkali-DU) clustered with the highly tolerant accessions IRGC 108921 and IRGC 26896 (Fig. 4). It is possible that the original Pokkali parent was heterozygous at this region and that different Pokkali alleles were selected when developing the RIL and NIL populations. Another possibility is that the original parent was homogeneous, but different plants were used to develop these populations. Further research is needed to investigate the origin of the Pokkali alleles in FL478 and the other lines, and to compare the tolerance mechanisms underlying these loci at Saltol and the other QTLs in the background.
The genetic relationships between seven Pokkali accessions (indicated by their IRGC accession numbers) and IR29, FL478, NIL-17, and NIL-30 are shown in (a) using 17 Saltol markers, and in (b) using 61 background SSR markers. Two gel pictures are provided as examples of multiple Pokkali alleles in (c) with marker RM8094 and in (d) with marker RM1287 for 24 DNA samples: lane 1 Nipponbare; lanes 2–8 Pokkali IRGC 8948, IRGC 15388, IRGC 15602, IRGC 15661, IRGC 28609, IRGC 26869, and IRGC 108921; lanes 11 and 12 IR29; lanes 13 and 14 FL478; lane 16 FL378; lanes 17–21 other RILs; and lanes 22–24 other NILs. A 25-bp DNA ladder is shown flanking the lanes, with sizes of 300, 275, 250, 225, 200, 175, and 150 bp from top to bottom for panel (c) and starting with 275 bp for panel (d). For marker RM8094, the FL478 allele appears to match the Pokkali accession IRGC 28609 at approximately 165 bp (c), while for RM1287, FL478 is monomorphic with IR29, but FL378 appears to match several Pokkali accessions at approximately 175 bp (d).
Marker-assisted backcrossing scheme for Saltol
While Saltol is a promising QTL for use in marker-assisted breeding for increased salinity tolerance, the presence of different Pokkali alleles identified in the Saltol region, in addition to the unknown relationship between Saltol and the other QTLs in the background, requires a careful strategy for transferring tolerant alleles into new varieties. To develop a complete MABC package for Saltol, markers across the region were first tested for polymorphism and robustness across several donors and potential recurrent parents. The best markers within the Saltol QTL region were AP3206, RM8094, and RM3412, the most useful markers flanking the Saltol region were RM1287 and RM10694 (telomeric to Saltol) and RM493 and RM10793 (centromeric to Saltol), while nearby markers that can be used for negative selection are RM490 above Saltol and RM562 and RM7075 below (Supplementary Table 6). The initial MABC lines for Saltol were developed using FL478 as the donor, due to its high level of tolerance, but without the tallness, photoperiod sensitivity, and late flowering of the original Pokkali landrace. Several MABC lines have now been developed at IRRI with the FL478 Saltol allele in the background of several popular varieties, and the introgression lines showed an increased level of tolerance, but not as high as the original FL478 donor (unpublished data). Since there may be differing effects from the multiple Pokkali alleles at Saltol, MABC lines are also being developed at Dhaka University and Bangladesh Rice Research Institute (BRRI), with the more common Pokkali Saltol allele found in RIL FL378, which is being transferred into two popular varieties from Bangladesh, BR11 and BRRI dhan 28 (unpublished data). Further testing is necessary to determine the relative tolerance effect between these two Saltol alleles, as well as the ultimate effect this seedling stage tolerance will have on crop establishment and grain yield under farmer’s conditions, particularly in areas where salinity is high at the beginning of the season such as in coastal areas during the monsoon season. In addition, once other Pokkali-derived QTLs besides Saltol are better characterized, these can be precisely combined with Saltol using MABC in a QTL pyramiding scheme to increase the levels of salt tolerance, while simultaneously avoiding the effects of negative linkage drag through the use of small introgression sizes.
Discussion
The original IR29/Pokkali QTL study using 80 extreme RILs identified Saltol as the QTL with the highest significance for shoot Na–K ratio with an LOD of 14.5 and R2 of 64%, based on selective genotyping (Gregorio 1997). A follow-up study categorized the RILs into sensitive and tolerant groups and mapped the position of Saltol between RM23 and RM140 (10.7–12.2 Mb on chromosome 1), and confirmed the effect of the shoot Na–K ratio with an LOD of 6.6 and R2 of 43% using 54 RILs (Bonilla et al. 2002). While neither of these studies presented the percent variation explained for visual SES tolerance scores or survival, it was assumed that by controlling the key mechanism of Na+/K+ homeostasis under stress, Saltol is a major contributor to seedling stage tolerance. The data from the current study confirmed that Saltol contributes to Na+/K+ homeostasis with an LOD of 7.6 and R2 of 27% across the 140 RILs and a 30% decrease in the shoot Na–K ratio, from 1.7 to 1.2 in the IR29/Pokkali backcross lines, while the Saltol effect on SES scores in the QTL population and backcross lines was much smaller. The fact that Saltol affected the Na–K ratio more than other traits supports the possibility that the sodium transporter SKC1 (OsHKT1;5 as in Platten et al. 2006) may be the causal gene underlying the Saltol QTL. SKC1 was found to encode a sodium transporter that helps control Na+/K+ homeostasis through unloading of Na+ from the xylem (Ren et al. 2005), which has been suggested to function primarily in roots to reduce the amount of Na+ ions that are transported to the leaves (Hauser and Horie 2010). Although the SKC1 QTL was originally detected using Nona Bokra, more research is needed to characterize the Pokkali allele at SKC1 to determine if it serves a similar function to maintain Na+/K+ homeostasis in the shoots. Interestingly, a recent study identified a QTL for Na–K ratio between 11.1 and 14.6 Mb on chromosome 1 from the upland japonica variety Moroberekan (Haq et al. 2010) suggesting that the Saltol region may have functional significance for salt tolerance across both indica and japonica varieties.
While Saltol explains much of the effect towards maintaining a healthy Na–K ratio under salt stress, it does not by itself provide a high degree of salt tolerance, as measured by visual scores of leaf symptoms. For example, none of the Saltol NILs and backcross lines could reach the high level of tolerance seen in FL478. Conversely, several NILs without a Pokkali allele at Saltol still exhibited higher tolerance than the IR29 check (Figs. 2 and 3), which suggests that additional Pokkali-derived QTLs are involved in providing tolerance. Similar results were also seen in a recent study using IR29/Pokkali backcross lines, where non-Saltol lines averaged an SES score of 6.3 compared to IR29 at SES 9 under salt stress of 18 dS m−1 (Alam et al. 2010). That study used single marker analysis on 65 SSR markers with 181 BC3F4 individuals to map QTLs for SES on chromosomes 3, 5, 6, 10, and 11, albeit in different regions than the current study, except for a QTL in common on chromosome 3 at RM3867. NIL development for additional Pokkali-derived QTLs is needed to investigate which tolerance mechanisms are controlled by these additional QTLs and if they work in an additive manner in combination with Saltol.
Another key finding in this study is that multiple Pokkali alleles were found at the Saltol locus between different RILs in the original mapping population. Subsequently, backcross lines with multiple Pokkali alleles were developed to further investigate the allelic variation at Saltol. In addition to the two major alleles found in the RIL population, as seen in FL540 and FL378 versus the distinct allele in FL478, two additional alleles were seen in the backcross lines developed from the families NIL-17 and NIL-30 (Fig. 3). Although the marker patterns differed across the Saltol region, a gene-based marker at SKC1 revealed that the NIL-17 line was similar to the common Pokkali allele, while the NIL-30 line shared an allele with IR29 at that marker locus, i.e. they do not have the Saltol locus. Progeny from these two families were further characterized for expression of candidate genes underlying Saltol, revealing that the SKC1 gene transcripts increased in Pokkali and NIL-17 at 7 and 14 days after salinization, but not in NIL-30, FL478, or IR29 (Tumimbang-Raiz and Blumwald, unpublished data). Sequencing of the SKC1 alleles across the different NILs may help identity the origin of the different Pokkali alleles and would allow for characterization of any functional polymorphisms that may contribute to the Saltol phenotype. Although the presence of multiple Pokkali alleles has complicated the validation and application of the Saltol locus, it also presents an opportunity to further investigate the effect of allelic variation from different Pokkali alleles on the underlying tolerance mechanisms.
While Saltol is a promising QTL for seedling stage salinity tolerance, its specific role in controlling Na+/K+ homeostasis and the presence of different Pokkali alleles in the Saltol region calls for a careful strategy for transferring tolerant alleles into new varieties as part of an integrated molecular breeding program. Unlike the success of using the Sub1 QTL for molecular breeding, where a single allele at a major locus can provide submergence tolerance (Neeraja et al. 2007; Septiningsih et al. 2009), the Saltol locus will likely be just one component of a multifaceted strategy to improve rice yields on salt affected soils. The initial steps have been taken to use two donors for the MABC transfer of different Saltol alleles into popular varieties to test the efficacy of these alleles to provide seedling stage tolerance: FL478 at IRRI and FL378 at Dhaka University and BRRI. Since MABC can transfer a QTL with minimal linkage drag, it is best to choose recurrent parents that have already been accepted by farmers, such as the mega-varieties that are cultivated on over 1 million hectares, so that the new MABC products will be more rapidly adopted. However, it can be difficult to predict whether a QTL will have the same tolerance effect in a different genetic background until a MABC line is developed in the new background. Even with validation of the donor varieties and closely linked markers, there are still uncertainties regarding the genetic background effects, epistasis, and QTL × environment interactions. Therefore, after the MABC lines are completed using marker selection, their level of tolerance needs to be confirmed under greenhouse and field conditions before amplifying seed for large-scale testing and validation in farmers’ fields at target areas.
Another major challenge is that seedling stage and reproductive stage salt tolerance are controlled by different mechanisms (Moradi et al. 2003); therefore, QTLs for both traits will need to be combined to provide a complete solution for the variable salt stress conditions in farmer’s fields (Ismail et al. 2008, 2010). Likewise, if Saltol primarily functions to regulate the uptake of Na+ and K+ and hence the Na–K ratio in shoot tissue, additional QTLs may be needed to contribute to other mechanisms for seedling stage tolerance, such as those controlling sequestration of sodium in vacuoles to provide increased tissue tolerance, by storing salts in old tissues and roots, and by triggering the mechanisms involved in protection against reactive oxygen species and chlorophyll degradation (Blumwald et al. 2000; Ismail et al. 2007; Moradi and Ismail 2007). Thus, more research is needed to characterize additional QTLs for both seedling and reproductive stage tolerance, and to validate the most potent combinations of QTLs for each stress-prone environment. Therefore, the long-term goal is to identify and combine genes and QTLs controlling different physiological mechanisms at both the seedling and reproductive stages to rapidly develop rice varieties that can produce higher and more stable yields under high salt stress conditions.
Methods
Analysis of QTLs derived from IR29/Pokkali population
The original RIL population was derived from a cross of IR29 with “Pokkali B” made at IRRI in 1992 (IR66946). Subsequently, seeds of the Pokkali parent used in the cross were deposited in the IRRI Genebank in 2003 (IRGC 108921). The original population consisted of 276 F8 RILs (Gregorio 1997), and a subset of 140 lines was selected for the current study. The population was evaluated for seedling stage salinity tolerance using a hydroponic system under controlled conditions of 29/21°C day/night temperature, natural sunlight and 70% RH in the IRRI phytotron in April 2006 (Experiment 1) and November 2006 (Experiment 2). Pre-germinated seeds were sown in holes on styrofoam floats with a net bottom suspended on trays filled with Yoshida nutrient solution (Yoshida et al. 1976). Three replications were used for each experiment, with nine individual plants per line evaluated for each replication. For Experiment 1, salt stress was imposed 14 days after germination by adding NaCl to an EC of 12 dS m−1 in Yoshida nutrient solution until final scoring. For Experiment 2, 5-day-old seedlings were salinized to EC 6 dS m−1 for 5 days, then EC 12 dS m−1 for 5 days, followed by EC of 18 dS m−1 until the final scoring. IR29 (sensitive) and FL 478 (highly tolerant) were used as checks. The pH of the nutrient solution was adjusted daily to 5.0, and the culture solutions were replaced weekly. Entries were scored based on visual symptoms using IRRI’s SES for rice, with ratings from 1 (highly tolerant) to 9 (highly sensitive; IRRI 1996). In addition, Na+ and K+ concentrations in leaves and roots, seedling height and chlorophyll content in leaves were assessed.
Marker genotype data was obtained by running SSR markers using 15 μL PCR reactions on 96-well plates with a 55 C annealing temperature (MJ Research and G-Storm thermal cyclers), and then run on 6% acrylamide gels at 100 v (Dual Triple-Wide Mini-Vertical System, C.B.S. Scientific, CA, USA) followed by SYBR-Safe staining (Invitrogen), gel documentation (Alpha Innotech), and manual scoring of the gel pictures. Out of the 140 lines, 14 RILs had greater than 10% heterozygous loci, which were converted to missing data before the analysis. Any extra alleles that did not match the IR29 parent were assumed to be of Pokkali origin and were designated as Pokkali alleles for the analysis. The molecular map of the 100 SSRs was based on the order of their position on the physical map based on the Nipponbare genomic sequence (www.gramene.org), and the marker distances were calculated by multiplying the Mb positions by a factor of 4 to obtain an equivalent estimate of cM (Supplementary Table 1). The data of 140 RILs with 100 SSRs and 14 traits was analyzed by interval mapping using QGene v4.3.6 (http://coding.plantpath.ksu.edu/qgene/). Permutation tests were performed for each trait with interval mapping and 10,000 permutations. On average, the 0.05 significance level was LOD 2.75 across all traits, which was then used as the threshold for declaring a significant QTL. Composite interval mapping was performed, and no additional QTLs were detected. Therefore, only the interval mapping results are presented. For comparison, an alternate analysis was performed by calculating the linkage map using MapManagerQTX and exporting into QTL Cartographer format for interval mapping analysis using Windows QTL Cartographer v2.5 (http://statgen.ncsu.edu/qtlcart/WQTLCart.htm). The QTL results were similar between the two programs, although QTL Cartographer indicated higher effects at several loci (Tables 1 and 2).
IR29/Pokkali and FL478/IR29 backcross lines
A set of 39 BC3F5 IR29/Pokkali lines were developed at IRRI by backcrossing IR29/Pokkali F1 plants to IR29, and screening BC1F1 and BC2F1 populations with 40 SSRs to select for the Saltol region and reduce the number of Pokkali introgressions in the background (Niones 2004). Selfed progeny from each backcross line were evaluated under salinity stress at the seedling stage in two experiments as described above for the RIL population. The average SES score, shoot and root Na–K ratios and leaf chlorophyll content were tested by comparing the means of the Saltol lines with a homozygous Pokkali allele versus the non-Saltol lines with an IR29 allele using a t-test at p < 0.05 to determine if the Saltol effect was significant (Supplementary Table 2).
To develop backcross lines with different Pokkali introgressions across the Saltol region, approximately 500 individuals from seven FL478/IR29 BC3F4 backcross families were screened with three SSR markers (RM490, RM10793 and RM7075) and 300 individuals from eight BC3F5 IR29/Pokkali lines were genotyped with six SSR markers (RM490, RM1287, RM10793, RM10864, RM7075, and RM24) to select for new recombinants in the Saltol region and for fixed lines representative of different Pokkali introgressions across the region. Subsequently, 71 backcross lines and their progeny were tested for seedling stage tolerance in a hydroponic screen with three replications in the IRRI phytotron in August 2007. Seedlings were sown on Yoshida nutrient solution and salinized to EC of 6 dS m−1 after 12 days, increased 3 days later to 12 dS m−1, and scored for visual tolerance (SES) 24, 31, and 35 days after salinization. Genotyping was performed with 38 markers and a comparison was made with 16 backcross lines and three RILs (Fig. 3). A second experiment was performed in two indoor growth chambers in April 2008: one at the standard 29°C/21°C day/night temperature regime, while a second was set at 40°C/30°C to simulate high stress environments. Salinization of 5-day-old seedlings was started at EC 12 dS m−1 for 1 week, followed by EC 18 dS m−1 until final scoring 2 weeks later for SES and Na+/K+ measurements (Supplementary Table 3).
FL478-derived Saltol NILs were developed as follows: 140 FL478/IR29 BC3F4 lines were genotyped with 28 SSR markers and 55 selected lines were screened for tolerance in the phytotron. Five lines were backcrossed two more times to IR29 and 122 FL478/IR29 BC5F1 plants were genotyped with 63 SSR markers. Nine NILs with a single Pokkali introgression at the Saltol QTL on chromosome 1 containing the FL478 allele between RM10694 at 11 Mb and RM10793 at 12.5 Mb, and a clean IR29 background (lines B3, B6, B7, B19, B24, C19, D7, D10, and F5) were identified and their progeny were grown in saline fields in Iloilo, Philippines in the 2009 wet season for yield testing, along with the IR29 as a check and an FL478/IR29 BC5F2 family (A18) with 11 additional Pokkali introgressions in the background (Supplementary Table 4). The yield trial was replicated three times using a randomized complete block design. Initial salinity (electrical conductivity of saturated extract) of the field during transplanting was as high as 11.99 dS m−1, which then decreased with the onset of rain to reach 7.75 dS m−1 at flowering and 6.68 dS m−1 at harvest. Since Saltol confers tolerance at the seedling stage, the initial high salinity at transplanting was useful to evaluate the performance of the NILs at this stage.
Developing Saltol-specific markers
Marker saturation of the region was performed by incorporating new SSRs from the IRGSP and custom-designed insertion/deletion (indel) markers at gene loci across the QTL peak region from 10.7 to 12.5 Mb. The annotated genes within the Saltol QTL region were inspected using the online TIGR Rice Genome Browser and several loci were targeted for developing gene-based PCR markers, including SKC1, SalT, and a pectinesterase gene (Supplementary Table 6).
Characterizing multiple Pokkali alleles
A comparison was performed with 17 markers across the Saltol region and 61 SSRs across the background on seven Pokkali accessions (IRGC 8948, 15388, 15602, 15661, 26896, 28609, 108921) and IR29, FL478, NIL-17, and NIL-30 by scoring allele sizes in base pairs for each gel picture. The data were run with PowerMarker software (http://statgen.ncsu.edu/powermarker/) using the Shared Allele function in a UPGMA dendrogram (Supplementary Table 5). NIL-17 and NIL-30 were derived from BC3 of IR29/Pokkali. The seven Pokkali accessions were also phenotyped in the same seedling stage screen as the backcross lines (August 2007) and showed average SES scores of: IRGC 15388 = 4.9, IRGC 26869 = 4.9, IRGC 108921 = 5.0, IRGC 28609 = 5.8, IRGC 15661 = 6.2, IRGC 8948 = 7.0, IRGC 15602 = 7.2.
Determining concentrations of sodium and potassium in root and shoot and chlorophyll in leaves
Root and shoot samples were collected and washed thoroughly, and dried at 70°C. Samples were then ground to a fine powder, and 10 mg were extracted in 10 ml of 0.1 N acetic acid in a water bath at 90°C for at least 2 h. The extracted tissue was cooled at room temperature, and the evaporated solution replaced with 0.1 N acetic acid. Samples were diluted 10 times, and Na+ and K+ concentrations were measured using an atomic absorption spectrophotometer (AAS 3100, Perkin Elmer, USA). For chlorophyll content, leaf samples were collected and immediately frozen in liquid nitrogen, freeze dried, and ground to a fine powder and used for total chlorophyll determination (Mackinney 1941).
References
Akbar M, Yabuno T, Nakao S. Breeding for saline resistant varieties of rice. I. Variability for salt tolerance among some rice varieties. Jpn J Breed. 1972;22:277–84.
Alam R, Rahman MS, Seraj ZI, Thomson MJ, Ismail AM, Tumimbang E et al. Investigation of seedling-stage salinity tolerance QTLs using backcross lines derived from Oryza sativa L. Pokkali. Plant Breed 2010; (in press).
Blumwald E, Aharon GS, Apse MP. Sodium transport in plant cells. Biochem Biophys Acta. 2000;1465:140–51.
Bohnert HJ, Gong Q, Li P, Ma S. Unraveling abiotic stress tolerance mechanisms—getting genomics going. Curr Opin Plant Biol. 2006;9:180–8.
Bonilla P, Dvorak J, Mackill D, Deal K, Gregorio G. RLFP and SSLP mapping of salinity tolerance genes in chromosome 1 of rice (Oryza sativa L.) using recombinant inbred lines. Philipp Agric Sci. 2002;85:68–76.
Chinnusamy V, Schumaker K, Zhu J-K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signaling in plants. J Exp Bot. 2004;55:225–36.
Flowers TJ, Yeo AR. Variability in the resistance of sodium chloride salinity within rice (Oryza sativa L.) varieties. New Phytol. 1981;88:363–73.
Garciadeblás B, Senn ME, Banuelos A, Rodriguez-Navarro A. Sodium transport and HKT transporters: the rice model. Plant J. 2003;34:788–801.
Gregorio GB. Tagging salinity tolerance genes in rice using amplified fragment length polymorphism (AFLP). PhD. thesis, University of the Philippines, Los Baños. 1997; 118 p.
Gregorio GB, Senadhira D, Mendoza RD, Manigbas NL, Roxas JP, Guerta CQ. Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res. 2002;76:91–101.
Haq TU, Gorham J, Akhtar J, Akhtar N, Steele KA. Dynamic quantitative trait loci for salt stress components on chromosome 1 of rice. Funcl Plant Biol. 2010;37:634–45.
Hauser F, Horie T. A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010;33:552–65.
Horie T, Schroeder JI. Sodium transporters in plants: diverse genes and physiological functions. Plant Physiol. 2004;136:2457–62.
Ismail AM, Heuer S, Thomson MJ, Wissuwa M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol Biol. 2007;65:547–70.
Ismail AM, Thomson MJ, Singh RK, Gregorio GB, Mackill DJ. Designing rice varieties adapted to coastal areas of South and Southeast Asia. J Indian Soc Coast Agric Res. 2008;26:69–73.
Ismail AM, Thomson MJ, Vergara GV, Rahman MA, Singh RK, Gregorio GB, et al. Designing resilient rice varieties for coastal deltas using modern breeding tools. In: Hoanh CT, Szuster BW, Pheng KS, Ismail AM, Nobel AD, editors. Tropical Deltas and coastal zones: food production, communities and environment at the land-water interface. Wallingford: CAB; 2010. p. 154–65.
IRRI. Standard evaluation system for rice. 4th ed. Manila: International Rice Research Institute; 1996. p. 52.
Kim S-H, Bhat PR, Cui X, Walia H, Xu J, Wanamaker S, et al. Detection and validation of single feature polymorphisms using RNA expression data from a rice genome array. BMC Plant Biol. 2009;9:65. doi:10.1186/1471-2229-9-65.
Lin HX, Zhu MZ, Yano M, Gao JP, Liang ZW, Su WA, et al. QTLs for Na+ and K+ uptake of the shoots and roots controlling rice salt tolerance. Theor Appl Genet. 2004;108:253–60.
Mackinney G. Absorption of light by chlorophyll solutions. J Biological Chem. 1941;140:315–22.
Martinoia E, Maeshima M, Neuhaus HE. Vacuolar transporters and their essential role in plant metabolism. J Exp Bot. 2007;58:83–102.
Mäser P, Gierth M, Schroeder JI. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil. 2002;247:43–54.
Moradi F, Ismail AM. Responses of photosynthesis, chlorophyll fluorescence and ROS scavenging system to salt stress during seedling and reproductive stages in rice. Ann Bot. 2007;99:1161–73.
Moradi F, Ismail AM, Gregorio GB, Egdane JA. Salinity tolerance of rice during reproductive development and association with tolerance at the seedling stage. Ind J Plant Physiol. 2003;8:105–16.
Munns R, Tester M. Mechanisms of salinity tolerance. Ann Rev Plant Biol. 2008;59:651–81.
Nakayama H, Horie T, Yonamine I, Shinmyo A, Yoshida K. Improving salt tolerance in plant cells. Plant Biotechnol. 2005;22:477–87.
Neeraja CN, Maghirang-Rodriguez R, Pamplona A, Heuer S, Collard BCY, Septiningsih EM, et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor Appl Genet. 2007;115:767–76.
Niones JM. Fine mapping of the salinity tolerance gene on chromosome 1 of rice (Oryza sativa L.) using near isogenic lines. MS dissertation. Laguna: University of the Philippines Los Baños; 2004.
Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn DJ, et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 2006;11:372–4.
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, et al. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nature Genet. 2005;37:1141–6.
Rodriguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. J Exp Bot. 2006;57:1149–60.
Sahi C, Singh A, Kumar K, Blumwald E, Grover A. Salt stress response in rice: genetics, molecular biology, and comparative genomics. Funct Integr Genomics. 2006;6:263–84.
Senadheera P, Maathuis FJM. Differentially regulated kinases and phosphatases in roots may contribute to inter-cultivar difference in rice salinity tolerance. Plant Signl Behavior. 2009;4:1163–5.
Senadheera P, Singh RK, Maathuis FJM. Differentially expressed membrane transporters in rice roots may contribute to cultivar dependent salt tolerance. J Exp Bot. 2009;60:2553–63.
Septiningsih EM, Pamplona AM, Sanchez DL, Maghirang-Rodriguez R, Neeraja CN, Vergara GV, et al. Development of Submergence-tolerant rice cultivars: the Sub1 locus and beyond. Ann Bot. 2009;103:151–60.
Shylaraj KS, Sasidharan NK. VTL 5: a high yielding salinity tolerant rice variety for the coastal saline ecosystems of Kerala. J Trop Agric. 2005;43:25–8.
Singh RK, Flowers TJ. The physiology and molecular biology of the effects of salinity on rice. In: Pessarakli M, editor. Handbook of plant and crop stress. 3rd ed. Florida: Taylor and Francis; 2010. p. 901–42.
Singh RK, Gregorio GB, Jain RK. QTL mapping for salinity tolerance in rice. Physiol Mol Biol Plants. 2007;13:87–99.
Singh RK, Redoña ED, Refuerzo L. Varietal improvement for abiotic stress tolerance in crop plants: special reference to salinity in rice. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee, editors. Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. New York: Springer; 2010. p. 387–415.
Thomson MJ, Ismail AM, McCouch SR, Mackill MJ. Marker assisted breeding. In: Pareek A, Sopory SK, Bohnert HJ, Govindjee, editors. Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. New York: Springer; 2010. p. 451–69.
Walia H, Wilson C, Condamine P, Liu X, Ismail AM, Zeng L, et al. Comparative transcriptional profiling of two contrasting rice genotypes under salinity stress during the vegetative growth stage. Plant Physiol. 2005;139:822–35.
Walia H, Wilson G, Ismail AM, Close TJ, Cui X. Comparing genomic expression patterns across plant species reveals highly diverged transcriptional dynamics in response to salt stress. BMC Genomics. 2009;10:398. doi:10.1186/1471-2164-10-398.
Yamaguchi Y, Blumwald E. Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci. 2005;10:615–20.
Yeo AR, Flowers TJ. Salinity resistance in rice (Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. Aust J Plant Physiol. 1986;13:161–73.
Yoshida S, Forno DA, Cock JK, Gomez KA. Laboratory manual for physiological studies of rice. Manila: International Rice Research Institute; 1976. p. 38.
Acknowledgments
We thank Rochelle Zantua for technical assistance with marker genotyping, and Macario del Valle and Ricardo Eugenio for assistance in the development of different populations and in salinity screening. This work was funded in part by the Generation Challenge Program (Competitive Project #2), the Challenge Program on Water and Food (Competitive Project #7) and the German Federal Ministry for Economic Cooperation and Development (BMZ).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Data of 140 RILs, 100 SSRs, and 14 traits for IR29/Pokkali QTL study in QGene format. (XLS 148 kb)
Supplementary Table 2
Phenotype and genotype data for 100 SSR markers on 39 BC3F5 lines. (XLS 176 kb)
Supplementary Table 3
SES scores and genotype data for 38 markers on 18 backcross lines and FL478 with allele sizes scored in basepairs. (XLS 259 kb)
Supplementary Table 4
Phenotype and genotype data for 63 markers on nine FL478 BC5F2 NILs and an additional backcross line. (XLS 142 kb)
Supplementary Table 5
Analysis of allelic variation between lines and Pokkali accessions using 61 background SSRs (section A) and 17 Saltol markers (section B). (XLS 37 kb)
Supplementary Table 6
Markers in and around the Saltol region on the short arm of chromosome 1. (XLS 17 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Thomson, M.J., de Ocampo, M., Egdane, J. et al. Characterizing the Saltol Quantitative Trait Locus for Salinity Tolerance in Rice. Rice 3, 148–160 (2010). https://doi.org/10.1007/s12284-010-9053-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12284-010-9053-8