Abstract
Background
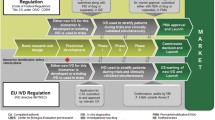
On July 1st, 2013, the Ministry of Health, Labor and Welfare (MHLW) issued an official notification regarding the co-development of companion diagnostics (CDx) with a drug which requires any exclusive diagnostic test or medical device to predict efficacy or adverse reactions to the drug. The main frame and contents in the MHLW’s notification are quite similar to the summaries in the final guidance issued by the US Food and Drug Administration (FDA) on August 6th, 2014 Guidance for Industry and Food and Drug Administration Staff (In Vitro Companion Diagnostic Devices, [2014] ), and these recommend industries to develop, study and submit CDx and the corresponding drug contemporaneously as much as possible. Following the MHLW’s notification, the Pharmaceutical and Medical Device Agency (PMDA) notified on December 26th, 2013, “the technical guidance for co-development of CDx and the drug” that mentioned the regulatory requirements for clinical trial of the drug and CDx as well as analytical validity of CDx required for the trials. These official notifications from the Ministry and the Agency may be useful for pharmaceutical and diagnostics makers to understand how they should co-develop and validate CDx for clinical trials and regulatory submission. However, since the most anticipated technologies such as the next generation sequencer (NGS) are more complex and its medical risks could be high level, the existing regulatory system focusing on only diagnostics reagents and devices that are developed and manufactured by in vitro diagnostics (IVD) makers may be no longer suitable for the characteristics of CDx for the future.As an increase of clinical needs for multiple biomarkers assay by DNA sequencer, on November 19th, 2013, the FDA cleared 510 K for NGS and its universal kit. On October 3rd, 2014, moreover, the agency notified two drafts of guidance (Anticipated Details of the Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratory in Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs), [2014]; Anticipated Details of the Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratory in FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDT), [2014]) for oversight of laboratory developed tests (LDTs) with medium or high medical risks. These FDA’s strategic decisions and new regulatory frameworks may allow the clinical laboratories to develop and perform more easily NGS-based CDx under the certification of Clinical Laboratory Improvement Amendments (CLIA). However, neither law nor regulated quality management system similar to the CLIA exists in Japan. To effectively validate LDTs and NGS for medical practice, Japan should learn more the current regulatory changes and initiatives in the US, as well as to reform the regulatory frameworks and create any regulated quality management system for clinical laboratory testing to be reimbursed.


Similar content being viewed by others
References
Guidance for Industry and Food and Drug Administration Staff. In Vitro Companion Diagnostic Devices, US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, August 6, 2014.
Lievre A, Bachet JB, Corre D, Boige V, Landi B, Emile JF, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5.
Ashraf N, Nishi Kothari N, Kim R. Predictive biomarkers for anti-epidermal growth factor receptor therapy: beyond KRAS testing. J Natl Compr Canc Netw. 2014;12:1433–42.
Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–80.
Miller I, Ashton‐Chess J, Spolders H, Fert V, Ferrara J, Werner Kroll W, et al. Market access challenges in the EU for high medical value diagnostic tests. Pers Med. 2011;8:137–48.
Hresko A, Haga SB. Insurance coverage policies for personalized medicine. J Pers Med. 2012;2:201–16.
Finch A, Lubinski J, Møller P, Singer C, Karlan B, Senter L, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1–8.
Anticipated Details of the Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratory. Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs), US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, October 31, 2014.
Anticipated Details of the Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratory. FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDT), US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, October 3, 2014.
Collins FS, Hamburg MA. First FDA authorization for next-generation sequencer. N Engl J Med. 2013;369:2369–71.
Conflict of interest
The author declares that he has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tazawa, Y. Perspective for the development of companion diagnostics and regulatory landscape to encourage personalized medicine in Japan. Breast Cancer 23, 19–23 (2016). https://doi.org/10.1007/s12282-015-0586-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-015-0586-y