Abstract
Background
Cyclin D1 (CCND1) gene amplification is a molecular key alteration in breast cancer and was suggested to predict resistance to antihormonal therapy. As tissue heterogeneity may affect diagnostic accuracy of predictive biomarkers, CCND1 genetic heterogeneity was assessed in this study. A novel tissue microarray (TMA) platform was manufactured for this purpose.
Methods
Primary breast carcinomas from 147 patients were sampled in a “heterogeneity-TMA” by taking eight different tissue cores from 4 to 8 tumor-containing blocks per case. Additional tissue samples were taken from 1 to 4 corresponding nodal metastases in 35 of these patients. CCND1 amplification was assessed by fluorescence in situ hybridization (FISH).
Results
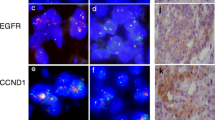
CCND1 amplification was seen in 28 of 133 (21.05 %) informative patients. Amplification was significantly associated with high tumor grade (p = 0.042), but unrelated to tumor type (p = 0.307), stage (p = 0.540) and ER (p = 0.061) or PR (p = 0.871) expression. A discordant Cyclin D1 amplification status was detected in 6 out of 28 (21.43 %) amplified tumors by heterogeneity-TMA analysis. Re-testing on large sections revealed three patients with true heterogeneity of high-level CCND1 amplification and another three patients with variable interpretation of borderline FISH ratios ranging between 1.7 and 2.3. No discrepancies were detected between 22 primary tumors and their matched lymph node metastases.
Conclusions
The high degree of homogeneity seen for CCND1 amplification suggests that this alteration is an early event in the development of a small subset of breast cancers.




Similar content being viewed by others
References
Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11(4):643–58. doi:10.1677/erc.1.00776.
Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456(7222):663–6. doi:10.1038/nature07483.
Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst. 2006;98(10):671–80. doi:10.1093/jnci/djj185.
Riggins RB, Lan JP, Zhu Y, Klimach U, Zwart A, Cavalli LR, et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68(21):8908–17. doi:10.1158/0008-5472.CAN-08-2669.
Shajahan AN, Riggins RB, Clarke R. The role of X-box binding protein-1 in tumorigenicity. Drug News Perspect. 2009;22(5):241–6. doi:10.1358/dnp.2009.22.5.1378631.
Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, et al. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5(6):597–605. doi:10.1016/j.ccr.2004.05.016.
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001;276(13):9817–24. doi:10.1074/jbc.M010840200.
Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, Lax S, et al. Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res. 2008;14(6):1767–74. doi:10.1158/1078-0432.CCR-07-4122.
Jirstrom K, Stendahl M, Ryden L, Kronblad A, Bendahl PO, Stal O, et al. Adverse effect of adjuvant tamoxifen in premenopausal breast cancer with cyclin D1 gene amplification. Cancer Res. 2005;65(17):8009–16. doi:10.1158/0008-5472.CAN-05-0746.
Bostner J, Ahnstrom Waltersson M, Fornander T, Skoog L, Nordenskjold B, Stal O. Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene. 2007;26(49):6997–7005. doi:10.1038/sj.onc.1210506.
Lundgren K, Holm K, Nordenskjold B, Borg A, Landberg G. Gene products of chromosome 11q and their association with CCND1 gene amplification and tamoxifen resistance in premenopausal breast cancer. Breast Cancer Res. 2008;10(5):R81. doi:10.1186/bcr2150.
Al-Kuraya K, Schraml P, Torhorst J, Tapia C, Zaharieva B, Novotny H, et al. Prognostic relevance of gene amplifications and coamplifications in breast cancer. Cancer Res. 2004;64(23):8534–40. doi:10.1158/0008-5472.CAN-04-1945.
Reis-Filho JS, Savage K, Lambros MB, James M, Steele D, Jones RL, et al. Cyclin D1 protein overexpression and CCND1 amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridisation analysis. Mod Pathol. 2006;19(7):999–1009. doi:10.1038/modpathol.3800621.
Elsheikh S, Green AR, Aleskandarany MA, Grainge M, Paish CE, Lambros MB, et al. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res Treat. 2006;109(2):325–35. doi:10.1007/s10549-007-9659-8.
Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res. 1999;5(8):2069–76.
Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA, Sutherland RL. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer. 2003;10(2):179–86.
Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, DiRenzo J, et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17(9):5338–47.
Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82(4):621–30.
Wilcken NR, Prall OW, Musgrove EA, Sutherland RL. Inducible overexpression of cyclin D1 in breast cancer cells reverses the growth-inhibitory effects of antiestrogens. Clin Cancer Res. 1997;3(6):849–54.
Stendahl M, Kronblad A, Ryden L, Emdin S, Bengtsson NO, Landberg G. Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer. 2004;90(10):1942–8. doi:10.1038/sj.bjc.6601831.
Hui R, Finney GL, Carroll JS, Lee CS, Musgrove EA, Sutherland RL. Constitutive overexpression of cyclin D1 but not cyclin E confers acute resistance to antiestrogens in T-47D breast cancer cells. Cancer Res. 2002;62(23):6916–23.
Petrakova K, Nenutil R, Grell P, Fabian P, Zichova I, Svoboda M, et al. Factors predicting failure of adjuvant hormonotherapy of breast carcinoma. A study in tamoxifen treated patients. Klin Onkol. 2008;21(5):303–8.
Muss HB, Bunn JY, Crocker A, Plaut K, Koh J, Heintz N, et al. Cyclin D-1, interleukin-6, HER-2/neu, transforming growth factor receptor-II and prediction of relapse in women with early stage, hormone receptor-positive breast cancer treated with tamoxifen. Breast J. 2007;13(4):337–45. doi:10.1111/j.1524-4741.2007.00440.x.
Ahnstrom M, Nordenskjold B, Rutqvist LE, Skoog L, Stal O. Role of cyclin D1 in ErbB2-positive breast cancer and tamoxifen resistance. Breast Cancer Res Treat. 2005;91(2):145–51. doi:10.1007/s10549-004-6457-4.
Han S, Park K, Bae BN, Kim KH, Kim HJ, Kim YD, et al. Cyclin D1 expression and patient outcome after tamoxifen therapy in estrogen receptor positive metastatic breast cancer. Oncol Rep. 2003;10(1):141–4.
Linke SP, Bremer TM, Herold CD, Sauter G, Diamond C. A multimarker model to predict outcome in tamoxifen-treated breast cancer patients. Clin Cancer Res. 2006;12(4):1175–83. doi:10.1158/1078-0432.CCR-05-1562.
Gillett C, Smith P, Gregory W, Richards M, Millis R, Peters G, et al. Cyclin D1 and prognosis in human breast cancer. Int J Cancer. 1996;69(2):92–9. doi:10.1002/(SICI)1097-0215(19960422)69:2<92:AID-IJC4>3.0.CO;2-Q.
Cuny M, Kramar A, Courjal F, Johannsdottir V, Iacopetta B, Fontaine H, et al. Relating genotype and phenotype in breast cancer: an analysis of the prognostic significance of amplification at eight different genes or loci and of p53 mutations. Cancer Res. 2000;60(4):1077–83.
Naidu R, Wahab NA, Yadav MM, Kutty MK. Expression and amplification of cyclin D1 in primary breast carcinomas: relationship with histopathological types and clinico-pathological parameters. Oncol Rep. 2002;9(2):409–16.
Seshadri R, Lee CS, Hui R, McCaul K, Horsfall DJ, Sutherland RL. Cyclin DI amplification is not associated with reduced overall survival in primary breast cancer but may predict early relapse in patients with features of good prognosis. Clin Cancer Res. 1996;2(7):1177–84.
Personeni N, Fieuws S, Piessevaux H, De Hertogh G, De Schutter J, Biesmans B, et al. Clinical usefulness of EGFR gene copy number as a predictive marker in colorectal cancer patients treated with cetuximab: a fluorescent in situ hybridization study. Clin Cancer Res. 2008;14(18):5869–76. doi:10.1158/1078-0432.CCR-08-0449.
Holst F, Stahl PR, Ruiz C, Hellwinkel O, Jehan Z, Wendland M, et al. Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nat Genet. 2007;39(5):655–60. doi:10.1038/ng2006.
Zaharieva BM, Simon R, Diener PA, Ackermann D, Maurer R, Alund G, et al. High-throughput tissue microarray analysis of 11q13 gene amplification (CCND1, FGF3, FGF4, EMS1) in urinary bladder cancer. J Pathol. 2003;201(4):603–8. doi:10.1002/path.1481.
Jensen LB, Bartlett JM, Witton CJ, Kirkegaard T, Brown S, Muller S, et al. Frequent amplifications and deletions of G1/S-phase transition genes, CCND1 and MYC in early breast cancers: a potential role in G1/S escape. Cancer Biomark. 2009;5(1):41–9. doi:10.3233/CBM-2009-0570.
Champeme MH, Bieche I, Hacene K, Lidereau R. Int-2/FGF3 amplification is a better independent predictor of relapse than c-myc and c-erbB-2/neu amplifications in primary human breast cancer. Mod Pathol. 1994;7(9):900–5.
Burkhardt L, Grob TJ, Hermann I, Burandt E, Choschzick M, Janicke F, et al. Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2010;9123(3):757–65. doi:10.1007/s10549-009-0675-8.
Simon R, Nocito A, Hubscher T, Bucher C, Torhorst J, Schraml P, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93(15):1141–6.
Jimenez RE, Wallis T, Tabasczka P, Visscher DW. Determination of Her-2/Neu status in breast carcinoma: comparative analysis of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2000;13(1):37–45. doi:10.1038/modpathol.3880007.
Masood S, Bui MM. Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci. 2000;30(3):259–65.
Watson PH, Safneck JR, Le K, Dubik D, Shiu RP. Relationship of c-myc amplification to progression of breast cancer from in situ to invasive tumor and lymph node metastasis. J Natl Cancer Inst. 1993;85(11):902–7.
Glockner S, Lehmann U, Wilke N, Kleeberger W, Langer F, Kreipe H. Amplification of growth regulatory genes in intraductal breast cancer is associated with higher nuclear grade but not with the progression to invasiveness. Lab Invest. 2001;81(4):565–71.
Moelans CB, de Weger RA, Monsuur HN, Maes AH, van Diest PJ. Molecular differences between ductal carcinoma in situ and adjacent invasive breast carcinoma: a multiplex ligation-dependent probe amplification study. Anal Cell Pathol (Amst). 2010;33(3):165–73. doi:10.3233/ACP-CLO-2010-0546.
Glockner S, Buurman H, Kleeberger W, Lehmann U, Kreipe H. Marked intratumoral heterogeneity of c-myc and cyclinD1 but not of c-erbB2 amplification in breast cancer. Lab Invest. 2002;82(10):1419–26.
Simon R, Nocito A, Hubscher T, Bucher C, Torhorst J, Schraml P, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93(15):1141–6.
Minner S, Gartner M, Freudenthaler F, Bauer M, Kluth M, Salomon G, et al. Marked heterogeneity of ERG expression in large primary prostate cancers. Modern Path. 2012;1:106–16. doi:10.1038/modpathol.2012.130.
Savic S, Tapia C, Grilli B, Rufle A, Bihl MP, De Vito Barascud A, et al. Comprehensive epidermal growth factor receptor gene analysis from cytological specimens of non-small-cell lung cancers. Br J Cancer. 2008;98(1):154–60. doi:10.1038/sj.bjc.6604142.
Tortola S, Steinert R, Hantschick M, Peinado MA, Gastinger I, Stosiek P, et al. Discordance between K-ras mutations in bone marrow micrometastases and the primary tumor in colorectal cancer. J Clin Oncol. 2001;19(11):2837–43.
Daniele L, Cassoni P, Bacillo E, Cappia S, Righi L, Volante M, et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J Thorac Oncol. 2009;4(6):684–8. doi:10.1097/JTO.0b013e3181a52359.
Monaco SE, Nikiforova MN, Cieply K, Teot LA, Khalbuss WE, Dacic S. A comparison of EGFR and KRAS status in primary lung carcinoma and matched metastases. Hum Pathol. 2010;41(1):94–102. doi:10.1016/j.humpath.2009.06.019.
Sauter G, Moch H, Moore D, Carroll P, Kerschmann R, Chew K, et al. Heterogeneity of erbB-2 gene amplification in bladder cancer. Cancer Res. 1993;53(10 Suppl):2199–203.
Marx AH, Burandt EC, Choschzick M, Simon R, Yekebas E, Kaifi JT, et al. Heterogenous high-level HER-2 amplification in a small subset of colorectal cancers. Hum Pathol. 2010;41(11):1577–85. doi:10.1016/j.humpath.2010.02.018.
Marx AH, Tharun L, Muth J, Dancau AM, Simon R, Yekebas E, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2010;40(6):769–77. doi:10.1016/j.humpath.2008.11.014.
Acknowledgments
The authors appreciate the excellent technical support of Christina Koop, Sylvia Schnöger and Sasha Eghtessadi.
Conflict of interest
The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Burandt and M. Grünert have contributed equally to this work.
About this article
Cite this article
Burandt, E., Grünert, M., Lebeau, A. et al. Cyclin D1 gene amplification is highly homogeneous in breast cancer. Breast Cancer 23, 111–119 (2016). https://doi.org/10.1007/s12282-014-0538-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-014-0538-y