Abstract
Background
Although triple-negative breast cancer (TNBC) with epidermal growth factor receptor (EGFR) expression has been extensively studied, few studies have simultaneously examined EGFR expression and EGFR gene amplification. Here, we examined the correlations of EGFR expression with EGFR gene amplification, EGFR-activating mutations, and the expression of components of the Akt pathway.
Methods
Tumor tissues were obtained from 84 patients with TNBC. We analyzed the expression of EGFR, phosphorylated Akt (p-Akt), phosphorylated mammalian target of rapamycin (p-mTOR), and other relevant proteins using immunohistochemistry. We also analyzed EGFR gene and chromosome 7 copy numbers by dual-color in situ hybridization. DNA was extracted from formalin-fixed paraffin-embedded samples. Analysis of EGFR gene-activating mutations was performed using the smart amplification process version 2 assay.
Results
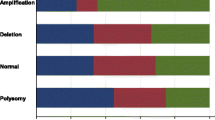
Most TNBCs expressing EGFR are non-specialized invasive ductal carcinomas, whereas others are likely to be rare specialized carcinomas, such as typical medullary carcinoma, apocrine carcinoma, metaplastic carcinoma, and adenoid cystic carcinoma. EGFR was expressed in samples from 28 of 84 (33.3 %) patients, but the EGFR gene was not amplified in any of the 84 samples. There were significant correlations between EGFR expression and the number of polysomic cells and the presence of high polysomy of chromosome 7. However, EGFR expression was not correlated with p-Akt or p-mTOR expression, nor with the other clinicopathological factors recorded in this study. We found no evidence of EGFR gene-activating mutations.
Conclusions
EGFR gene amplification and EGFR-activating mutations might not be the mechanisms leading to the constitutive activation of EGFR in TNBC. Further investigation is needed to clarify the other molecular mechanisms for oncogenic activation of EGFR in TNBC.



Similar content being viewed by others
References
Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92.
Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–28.
Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand–receptor interactions. FEBS Lett. 1997;410:83–6.
Liu W, Li J, Roth RA. Heregulin regulation of Akt/protein kinase B I breast cancer cells. Biochem Biophys Res Commun. 1999;261:897–903.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
McCubrey JA, Steelman LS, Chappel WH, Abrams SL, Wong EWT, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84.
Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–55.
John T, Liu G, Tsao MS. Overview of molecular testing in non-small-cell lung cancer: mutational analysis, gene copy number, protein expression and other biomarkers of EGFR for the prediction of response to tyrosine kinase inhibitors. Oncogene. 2009;28:S14–23.
Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–36. doi:10.1007/s10549-010-1293-1.
Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, et al. EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol. 2005;18:1027–33.
Reis-Filho JS, Pinheiro C, Lambros MB, Milanezi F, Carvalho S, Savage K, et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006;209:445–53.
Generali D, Leek R, Fox SB, Moore JW, Taylor C, Chambers P, et al. EGFR mutations in exons 18–21 in sporadic breast cancer. Ann Oncol. 2007;18:203–5.
Toyama T, Yamashita H, Kondo N, Okuda K, Takahashi S, Sasaki H, et al. Frequently increased epidermal growth factor receptor (EGFR) copy numbers and decreased BRCA1 mRNA expression in Japanese triple-negative breast cancers. BMC Cancer. 2008;8:309.
Teng YH, Tan WJ, Thike AA, Cheok PY, Tse GM, Wong NS, et al. Mutations in the epidermal growth factor receptor (EGFR) gene in triple negative breast cancer: possible implications for targeted therapy. Breast Cancer Res. 2011;13:R35. doi:10.1186/bcr2857.
Kersting C, Tidow N, Schmidt H, Liedtke C, Neumann J, Boecker W, et al. Gene dosage PCR and fluorescence in situ hybridization reveal low frequency of egfr amplifications despite protein overexpression in invasive breast carcinoma. Lab Invest. 2004;84:582–7.
Park K, Han S, Shin E, Kim HJ, Kim JY. EGFR gene and protein expression in breast cancers. Eur J Surg Oncol. 2007;33:956–60.
Gumuskaya B, Alper M, Hucumenoglu S, Altundag K, Uner A, Guler G. EGFR expression and gene copy number in triple-negative breast carcinoma. Cancer Genet Cytogenet. 2010;203:222–9.
The Japanese Breast Cancer Society. Histological classification of breast tumor. In: General rules for clinical and pathological recording of breast cancer. 16th ed. Tokyo: Kanehara; 2008; p. 18–24.
Ishikawa Y, Horiguchi J, Toya H, Nakajima H, Hayashi M, Tagaya N, et al. Triple-negative breast cancer: histological subtypes and immunohistochemical and clinicopathological features. Cancer Sci. 2011;102:656–62. doi:10.1111/j.1349-7006.2011.01858.x.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71.
Sasaki Y, Tsuda H. Clinicopathological characteristics of triple-negative breast cancers. Breast Cancer. 2009;16:254–9.
Tsuda H, Takarabe T, Hasegawa T, Murata T, Hirohashi S. Myoepithelial differentiation in high-grade invasive ductal carcinomas with large central acellular zones. Hum Pathol. 1999;30:1134–9.
Shousha S. Oestrogen receptor status of breast carcinoma: Allred/H score conversion table. Histopathology. 2008;53:346–7.
Nitta H, Hauss-Wegrzyniak B, Lehrkamp M, Murillo AE, Gaire F, Farrell M. Development of automated brightfield double in situ hybridization (BDISH) application for HER2 gene and chromosome 17 centromere (CEN 17) for breast carcinomas and an assay performance comparison to manual dual color HER2 fluorescence in situ hybridization (FISH). Diagn Pathol. 2008;3:41.
Mitani Y, Lezhava A, Kawai Y, Kikuchi T, Oguchi-Katayama A, Kogo Y, et al. Rapid SNP diagnostics using asymmetric isothermal amplification and a new mismatch-suppression technology. Nat Methods. 2007;4:257–62.
Hoshi K, Takakura H, Mitani Y, Tatsumi K, Momiyama N, Ichikawa Y, et al. Rapid detection of epidermal growth factor receptor mutations in lung cancer by the SMart-amplification process. Clin Cancer Res. 2007;13:4974–83.
Kawai Y, Kikuchi T, Mitani Y, Kogo Y, Itoh M, Usui K, et al. Sensitive detection of EGFR mutations using a competitive probe to suppress background in the SMart amplification process. Biologicals. 2008;36:234–8.
Rimawi MF, Shetty PB, Weiss HL, Schiff R, Osborne CK, Chamness GC, et al. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer. 2010;116:1234–42.
Francis GD, Jones MA, Beadle GF, Stein SR. Bright-field in situ hybridization for HER2 gene amplification in breast cancer using tissue microarrays: correlation between chromogenic (CISH) and automated silver-enhanced (SISH) methods with patient outcome. Diagn Mol Pathol. 2009;18:88–95.
García-García E, Gómez-Martín C, Angulo B, Conde E, Suárez-Gauthier A, Adrados M, et al. Hybridization for human epidermal growth factor receptor 2 testing in gastric carcinoma: a comparison of fluorescence in-situ hybridization with a novel fully automated dual-colour silver in-situ hybridization method. Histopathology. 2011;59:8–17. doi:10.1111/j.1365-2559.2011.03894.x.
Shin HJ, Shin DM, Tarco E, Sneige N. Detection of numerical aberrations of chromosomes 7 and 9 in cytologic specimens of pleural malignant mesothelioma. Cancer. 2003;99:233–9.
Li YH, Wang F, Shen L, Deng YM, Shao Q, Feng F, et al. EGFR fluorescence in situ hybridization pattern of chromosome 7 disomy predicts resistance to cetuximab in KRAS wild-type metastatic colorectal cancer patients. Clin Cancer Res. 2011;15(17):382–90. doi:10.1158/1078-0432.CCR-10-0208.
Buckingham LE, Coon JS, Morrison LE, Jacobson KK, Jewell SS, Kaiser KA, et al. The prognostic value of chromosome 7 polysomy in non-small cell lung cancer patients treated with gefitinib. J Thorac Oncol. 2007;2:414–22.
Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–23.
Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19:238–45.
Aleskandarany MA, Rakha EA, Ahmed MA, Powe DG, Ellis IO, Green AR. Clinicopathologic and molecular significance of phospho-Akt expression in early invasive breast cancer. Breast Cancer Res Treat. 2011;127:407–16. doi:10.1007/s10549-010-1012-y.
Acknowledgments
We thank Ms. A. Katayama (MD-PhD course student, Gunma University Faculty of Medicine), Dr. T. Hikino, Dr. M.Saito, and Mr. F. Hara (Department of Diagnostic Pathology, Gunma University Graduate School of Medicine) for their excellent technical assistance.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nakajima, H., Ishikawa, Y., Furuya, M. et al. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer. Breast Cancer 21, 66–74 (2014). https://doi.org/10.1007/s12282-012-0354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-012-0354-1